Effects of Adenosine Extract from Pholiota adiposa (Fr.) Quel on mRNA Expressions of Superoxide Dismutase and Immunomodulatory Cytokines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chromatographic Action of Extract from P. adiposa
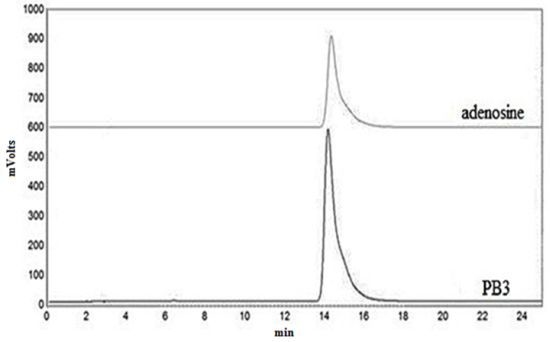
2.2. Identification of the Extract PB3
2.3. Effect of PB3 on mRNA Expression of SOD
2.4. Effect of PB3 on mRNA Expression of Immunomodulatory Cytokines
3. Experimental
3.1. Reagents
3.2. Animals
3.3. Sample Preparation and Isolation of Adenosine
3.4. Real-Time PCR
4. Conclusions
Acknowledgments
References
- Jagtap, S.S.; Dhiman, S.S.; Jeya, M.; Kang, Y.C.; Choi, J.H.; Lee, J.K. Saccharification of poplar biomass by using lignocellulases from Pholiota adipose. Bioresource Technol. 2012, 120, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Zhang, G.Q.; Zhou, B.; Lin, R.S.; Jia, L.; Fan, K.M.; Liu, X.N.; Wang, G.Y.; Wang, L.; Zhang, J.J. Extraction and in vitro antioxidant activity of intracellular polysaccharide by Pholiota adiposa SX-02. J. Biosci. Bioeng. 2011, 111, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ding, X.M.; Liu, H.Y.; Mi, Z.Q. Effect of crude polysaccharides of Pholiota adiposa on antitumor and immunity in bearing-tumor mice. Chin. Pharm. 2007, 10, 119–121. [Google Scholar]
- Dulger, B. Antimicrobial activity of the macrofungus Pholiota adipose. Fitoterapia 2004, 75, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Ely, S.W.; Berne, R.M. Protective Effects of Adenosine in Myocardial Ischemia. Circulation 1992, 85, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.J.; Liu, Z.Y. Effects of ischemic postconditioning and adenosine postconditioning on lung ischemia-reperfusion injury in rat. Mod. Med. J. 2009, 37, 196–199. [Google Scholar]
- Ramkumar, V.; Nie, Z.Z.; Rybak, L.P.; Maggirwar, S.B. Adenosine, antioxidant enzymes and cvtoprotection. Trends Pharmacol. Sci. 1995, 16, 283–285. [Google Scholar] [CrossRef]
- Schwantner, A.; Dingley, A.J.; Ozbek, S.; Rose-John, S.; Grötzinger, J. Direct determination of the interleukin-6 binding epitope of the interleukin-6 receptor by NMR spectroscopy. J. Biol. Chem. 2004, 279, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Braat, H.; Rottiers, P.; Hommes, D.W.; Huyghebaert, N.; Remaut, E.; Remon, J.P.; Deventer, S.J.; Neirynck, S.; Peppelenbosch, M.P.; Steidler, L. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clin. Gastroenterol. Hepatol. 2006, 4, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Molecular Weight Search Help. Available online: http://webbook.nist.gov/chemistry/mw-ser.html (accessed on 28 September 2011).
- Shryock, J.C.; Belardinelli, L. Adenosine and adenosine receptors in the cardiovascular system: Biochemistry, physiology and pharmacology. Am. J. Cardiol. 1997, 79, 2–10. [Google Scholar] [CrossRef]
- Headrick, J.P.; Peart, J.N.; Reichelt, M.E.; Haseler, L.J. Adenosine and its receptors in the heart: Regulation, retaliation and adaptation. BBA-Biomembranes 2011, 1808, 1413–1428. [Google Scholar] [CrossRef] [PubMed]
- Groux, H.; Cottrez, F. The complex role of interleukin-10 in autoimmunity. J. Autoimmun. 2004, 20, 281–285. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ng, T.B.; Liu, Z.K.; Wang, C.R.; Li, N.; Qiao, W.T.; Liu, F. Immunoregulatory and anti-HIV-1 enzyme activities of antioxidants components from lotus (Nelumbo nucifera Gaertn) rhizome. Bioscience Rep. 2010, 31, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound PB3 are available from the authors. |




| Gene | ΔCt * | ΔΔCt ** | 2−ΔΔCt |
|---|---|---|---|
| IL-2 | 9.43 ± 0.15 | 1.03 | 0.490 |
| IL-6 | 7.60 ± 0.19 | 0.81 | 0.569 |
| IL-10 | 8.05 ± 0.05 | −0.57 | 1.481 |
| IFN-γ | 7.81 ± 0.17 | 0.45 | 0.734 |
| SOD | 3.95 ± 0.17 | −0.70 | 1.621 |
| Gene | Sequence | Product | |
|---|---|---|---|
| SOD | upstream: | 5'-GCAGGGAACCATCCACTT-3' | 215 bp |
| downstream: | 5'-TGCCCAGGTCTCCAACAT-3' | ||
| IL-2 | upstream: | 5'-TGAACTTGGACCTCTGCG-3' | 220 bp |
| downstream: | 5'-AGGGCTTGTTGAGATGATGC-3' | ||
| IL-6 | upstream: | 5'-TTCTTGGGACTGATGCTG-3' | 380 bp |
| downstream: | 5'-CTGGCTTTGTCTTTCTTGTT-3' | ||
| IL-10 | upstream: | 5'-ACCAAAGCCACAAAGCAG-3' | 249 bp |
| downstream: | 5'-GGAGTCGGTTAGCAGTATG-3' | ||
| IFN-r | upstream: | 5'-TGAGACAATGAACGCTAC-3' | 142 bp |
| downstream: | 5'-TTCCACATCTATGCCACT-3' | ||
| GAPDH | upstream: | 5'-TCAACGGCACAGTCAAGG-3' | 470 bp |
| downstream: | 5'-ACCAGTGGATGCAGGGAT-3' | ||
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, C.R.; Qiao, W.T.; Zhang, Y.N.; Liu, F. Effects of Adenosine Extract from Pholiota adiposa (Fr.) Quel on mRNA Expressions of Superoxide Dismutase and Immunomodulatory Cytokines. Molecules 2013, 18, 1775-1782. https://doi.org/10.3390/molecules18021775
Wang CR, Qiao WT, Zhang YN, Liu F. Effects of Adenosine Extract from Pholiota adiposa (Fr.) Quel on mRNA Expressions of Superoxide Dismutase and Immunomodulatory Cytokines. Molecules. 2013; 18(2):1775-1782. https://doi.org/10.3390/molecules18021775
Chicago/Turabian StyleWang, Chang Rong, Wen Tao Qiao, Ye Ni Zhang, and Fang Liu. 2013. "Effects of Adenosine Extract from Pholiota adiposa (Fr.) Quel on mRNA Expressions of Superoxide Dismutase and Immunomodulatory Cytokines" Molecules 18, no. 2: 1775-1782. https://doi.org/10.3390/molecules18021775