Brazilin Inhibits Growth and Induces Apoptosis in Human Glioblastoma Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Brazilin
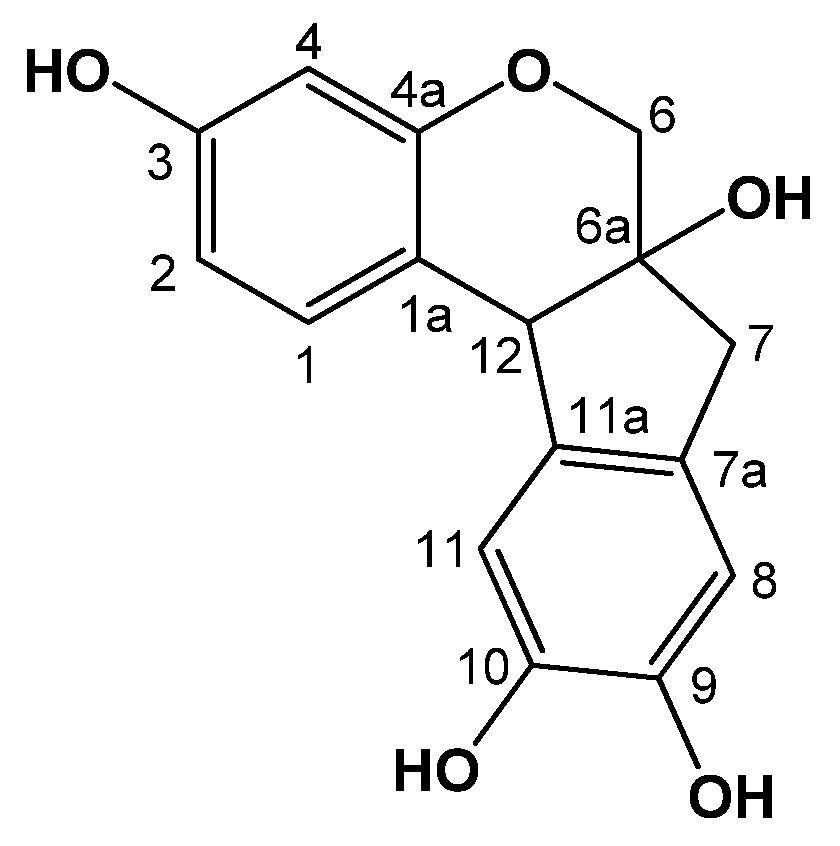
| No. | δH | δC | No. | δH | δC |
|---|---|---|---|---|---|
| 1 | 7.17 (1H, d, J = 8.4 Hz) | 137.3 | 7 | 3.07 (1H, d, J = 16 Hz, H-7a) | 42.7 |
| 2.74 (1H, d, J = 16 Hz, H-7b) | |||||
| 1a | 115.5 | 7a | 131.3 | ||
| 2 | 6.47 (1H, dd, J = 8.4, 2.4 Hz) | 109.9 | 8 | 6.60 (1H, s) | 112.8 |
| 3 | 6.90 (1H, d, J = 3.2 Hz) | 155.5 | 9 | 145.4 | |
| 4 | 6.31 (1H, d, J = 2.4 Hz) | 104.2 | 10 | 145.1 | |
| 4a | 157.6 | 11 | 6.71 (1H, s) | 112.4 | |
| 6 | 3.92 (1H, d, J = 11.2 Hz, H-6a) | 70.7 | 12 | 3.96 (1H, s) | 50.8 |
| 3.68 (1H, d, J = 11.2, Hz, H-6b) | |||||
| 6a | 78.0 |
2.2. Brazilin-Induced Cytotoxicity in Human Glioblastoma U87 Cells
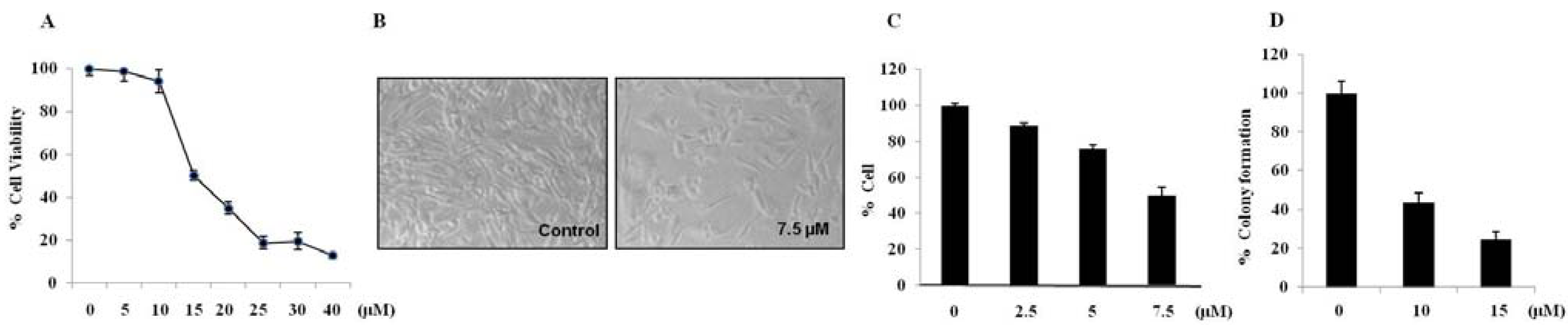
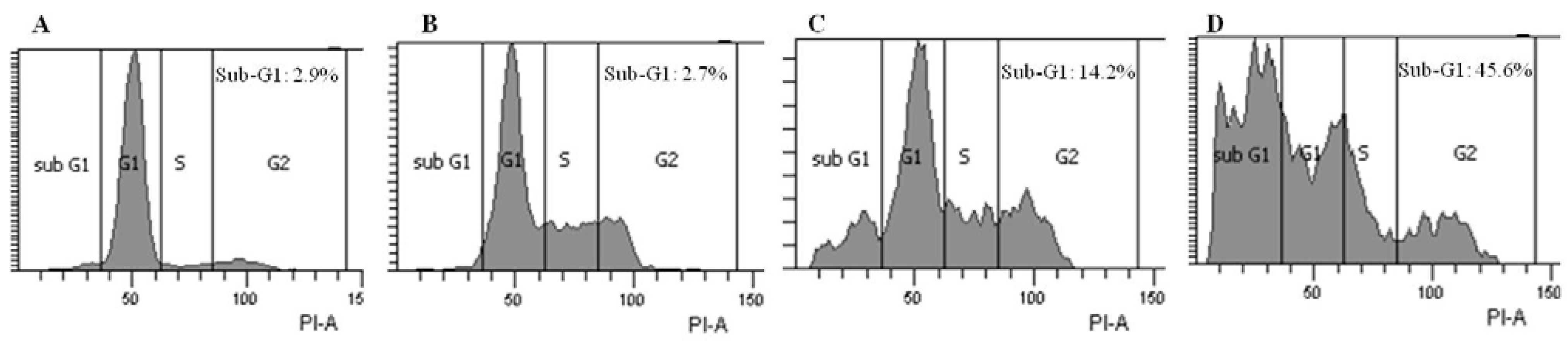
2.3. Brazilin-Induced High Accumulation of Sub-G1 Phase Cells
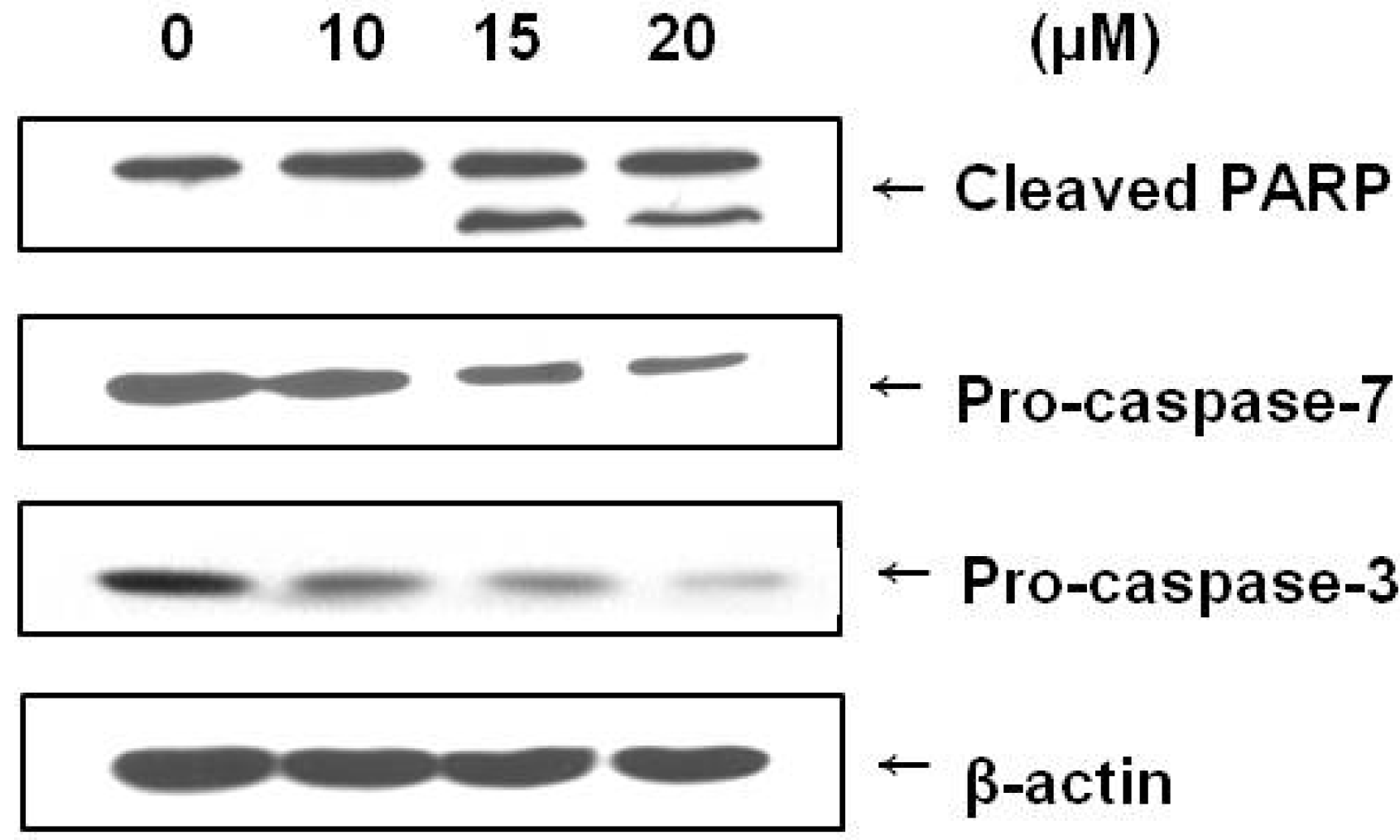
2.4. Brazilin Induced Activation of Caspase-3 and Caspase-7 and Cleavage of PARP
2.5. Discussion
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectroscopic Data
3.5. Cell Culture
3.6. MTT and Proliferation Assays
3.7. Morphological Observation
3.8. Soft Agar Assay
3.9. Flow Cytometric Analysis
3.10. Western Blot Analysis
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
References
- Robins, H.I.; Chang, S.; Butowski, N.; Mehta, M. Therapeutic advances for glioblastoma multiforme: Current status and future prospects. Curr. Oncol. Rep. 2007, 9, 66–70. [Google Scholar]
- Hu, J.; Jiang, C.; Ng, H.K.; Pang, J.C.; Tong, C.Y.; Chen, S. Genome-wide allelotype study of primary glioblastoma multiforme. Chin. Med. J. 2003, 116, 577–583. [Google Scholar]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of human brain tumour initiating cells. Nature 2004, 432, 396–401. [Google Scholar]
- Legler, J.M.; Ries, L.A.; Smith, M.A.; Warren, J.L.; Heineman, E.F.; Kaplan, R.S.; Linet, M.S. Cancer surveillance series [collected]: Brain and other central nervous system cancers: Recent treands in incidence and mortality. J. Natl. Cancer Inst. 1999, 16, 1382–1390. [Google Scholar]
- Hikino, H.; Taguchi, T.; Fujimura, H.; Hiramatsu, Y. Anti-inflammatory principles of Caesalpinia sappan wood and of Haematoxylon campechianum wood. Planta Med. 1977, 31, 214–220. [Google Scholar] [CrossRef]
- Ueda, J.Y.; Tezka, Y.; Banskota, A.H.; Le, T.Q.; Tran, Q.K.; Harimaya, Y.; Saiki, I.; Kadota, S. Anti-proliferative activity of Vietnamese medicinal plants. Biol. Pharm. Bull. 2002, 25, 753–760. [Google Scholar]
- Badami, S.; Moorkoth, S.; Rai, S.R.; Elango, K.; Suresh, B. Antioxidant activity of Caesalpinia sappan heartwood. Biol. Pharm. Bull. 2003, 26, 1534–1537. [Google Scholar] [CrossRef]
- Pan, C.; Zeng, X.; Guan, Y.; Jiang, X.; Li, L.; Zhang, H. Design and synthesis of brazilin-like compounds. Synlett 2011, 3, 425–429. [Google Scholar]
- Jadhav, U.; Ezhilarasan, R.; Vaughn, S.F.; Berhow, M.A.; Mohanam, S. Dietary isothiocyanate iberin inhibits growth and induces apoptosis in human glioblastoma cells. J. Pharmacol. Sci. 2007, 103, 247–251. [Google Scholar] [CrossRef]
- Zanotto-F, A.; Braganhol, E.; Edelweiss, M.I.; Behr, G.A.; Zanin, R.; Schroder, R.; Simoes-P, A.; Battastini, A.M.; Moreira, J.C. The curry spice curcumin selectively inhibits cancer cells growth in vitro and in preclinical model of glioblastoma. J. Nutr. Biochem. 2012, 23, 591–601. [Google Scholar] [CrossRef]
- Khan, M.; Yu, B.; Rasul, A.; Al, S.A.; Yi, F.; Yang, H.; Ma, T. Jaceosidin induces apoptosis in U87 glioblastoma cells through G2/M phase arrest. Evid. Based Complement Alternat. Med. 2012, 2012, 1–12. [Google Scholar]
- Fang, Y.; Christine, B.; Ralf, B.; Michael, H.; Renate, S.; Anna, S.; Anne, S.; Michael, J.; Richard, J. Sorafenib induced growth arrest and apoptosis of human glioblastoma cells through the dephosphorylation of signal transducers and activators of transcription 3. Mol. Cancer Ther. 2010, 9, 953–962. [Google Scholar]
- Kim, D.S.; Baek, N.I.; Oh, S.R.; Jung, K.Y.; Lee, I.S.; Lee, H.K. NMR assignment of brazilein. Phytochemistry 1997, 46, 177–178. [Google Scholar] [CrossRef]
- Ren, L.; Yang, X.; Wang, G.; Zhang, H.; Zhao, L.; Mi, Z. Inhibition effect of brazilin to human bladder cancer cell line T24. World Acad. Sci. Eng. Technol. 2011, 60, 215–219. [Google Scholar]
- Krakstad, C.; Crekenya, M. Survival signaling and apoptosis resistance in glioblastomas: Opportunities for targeted therapeutics. Mol. Cancer 2010, 9, 1–14. [Google Scholar]
- Woodle, E.S.; Kulkarni, S. Programmed cell death. Transplantation 1998, 66, 681–691. [Google Scholar] [CrossRef]
- Gerald, M.C. Caspases: The executioners of apoptosis. J. Biochem. 1997, 326, 1–16. [Google Scholar]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar]
- Sample Availability: Sample of the compound 1 is available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, D.-Y.; Lee, M.-K.; Kim, G.-S.; Noh, H.-J.; Lee, M.-H. Brazilin Inhibits Growth and Induces Apoptosis in Human Glioblastoma Cells. Molecules 2013, 18, 2449-2457. https://doi.org/10.3390/molecules18022449
Lee D-Y, Lee M-K, Kim G-S, Noh H-J, Lee M-H. Brazilin Inhibits Growth and Induces Apoptosis in Human Glioblastoma Cells. Molecules. 2013; 18(2):2449-2457. https://doi.org/10.3390/molecules18022449
Chicago/Turabian StyleLee, Dae-Young, Mi-Kyoung Lee, Geum-Soog Kim, Hyung-Jun Noh, and Min-Ho Lee. 2013. "Brazilin Inhibits Growth and Induces Apoptosis in Human Glioblastoma Cells" Molecules 18, no. 2: 2449-2457. https://doi.org/10.3390/molecules18022449
APA StyleLee, D.-Y., Lee, M.-K., Kim, G.-S., Noh, H.-J., & Lee, M.-H. (2013). Brazilin Inhibits Growth and Induces Apoptosis in Human Glioblastoma Cells. Molecules, 18(2), 2449-2457. https://doi.org/10.3390/molecules18022449




