Lanthanide-Mediated Dephosphorylation Used for Peptide Cleavage during Solid Phase Peptide Synthesis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Phosphorylated Resins
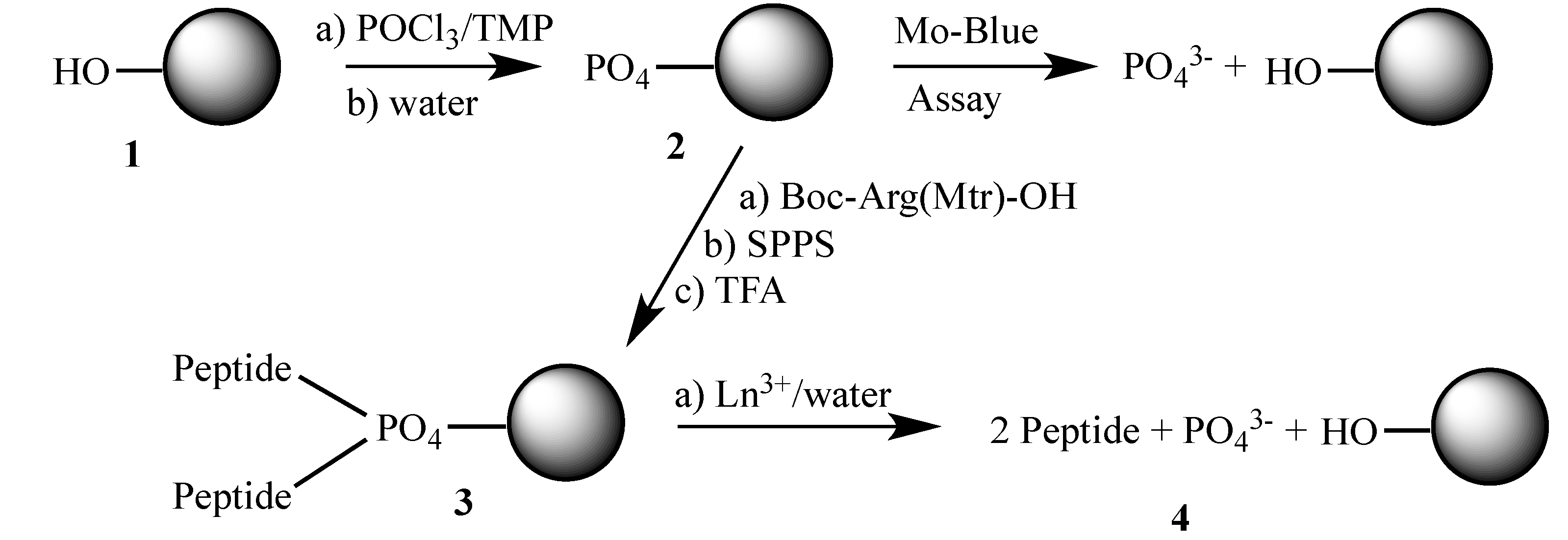
| Type of Phosphate | PO4 loading (mmol/g) |
|---|---|
| Pyrophosphate | 1.8 ± 1.4 1 |
| Acetylphosphate ester | 0.054 ± 0.022 |
| Phosphorous oxychloride | 0.41 ± 0.08 |
| Initial OH loading on resin | 1.20 |
2.2. Lanthanide-Mediated Dephosphorylation
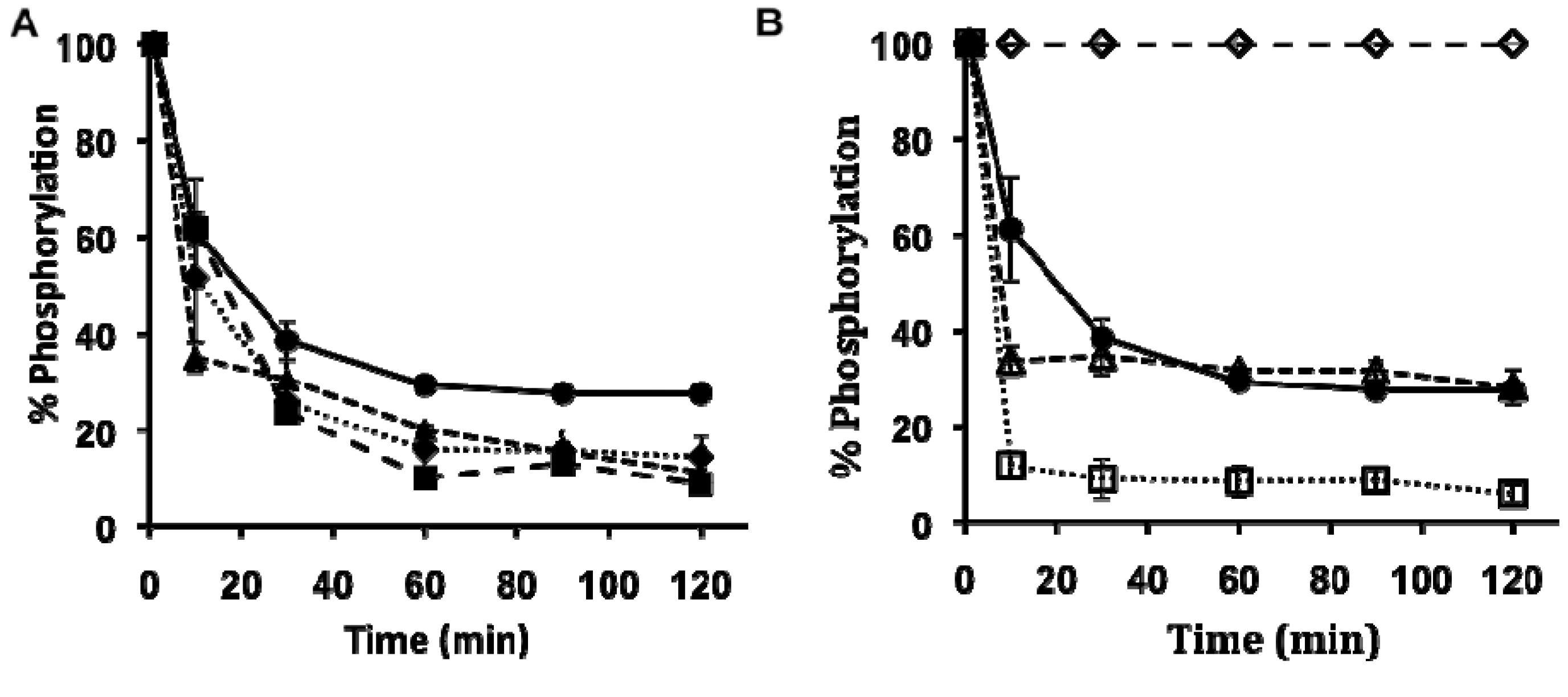
2.3. Peptide Synthesis

| Reactant | Reactant (μmol) | Cleavage Method | Peptide Product (μmol) | % Yield 1 |
|---|---|---|---|---|
| resin-bound phosphate | 65.2 | first Eu(III) | 51.6 | 79.1% |
| second Eu(III) | 15.0 | 23.0% | ||
| sum of 2 Eu(III) cleavages | 66.6 | 102.1%a | ||
| 99.2 | first Eu(III) | 71.2 | 71.8% | |
| second Eu(III) | 25.7 | 25.9% | ||
| sum of 2 Eu(III) cleavages | 96.9 | 97.7% | ||
| average of two trials of resin-bound phosphate | 82.2 ± 17 | sum of 2 Eu(III) cleavages | 81.8 ± 15.1 | 98.9 ± 1.2% |
| resin-bound hydroxyl groups | 77.0 | 5% water in TFA | 55.2 | 71.7% |
| 60.0 | 5% water in TFA | 58.4 | 97.3% | |
| average of two trials of resin-bound hydroxyl groups | 68.5 ± 8.5 | 5% water in TFA | 56.8 ± 1.6 | 84.5 ± 12.8% |

3. Experimental
3.1. General Procedures
3.2. Synthesis of Phosphate-Loaded Resins
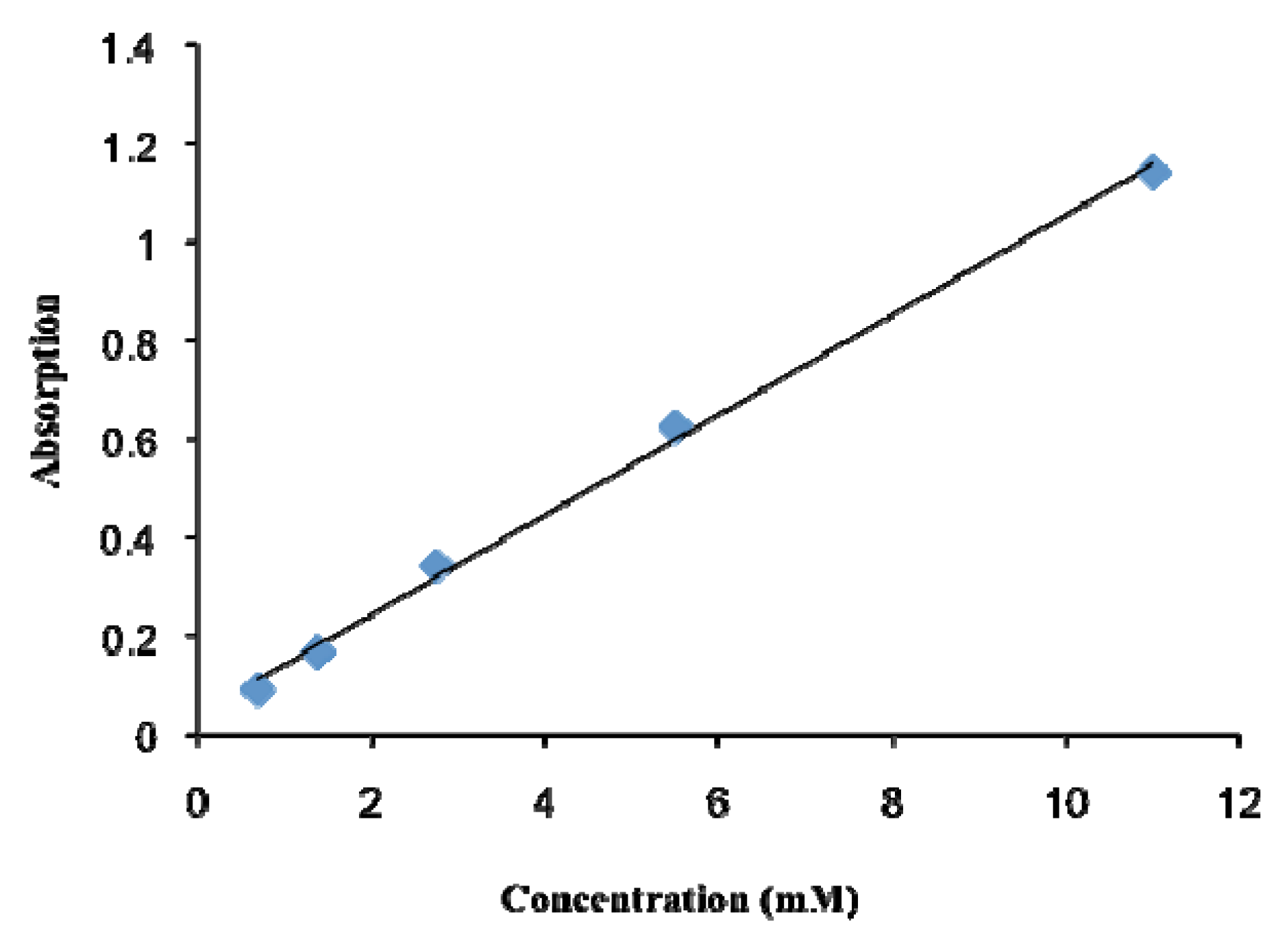
3.3. Quantification of the Phosphorylation of a Resin
3.4. Quantification of Phosphate Cleavage
3.5. Solid Phase Peptide Synthesis
3.6. Lanthanide-Mediated Peptide Cleavage
4. Conclusions
Acknowledgments
References
- Bracken, K.; Moss, R.A.; Ragunathan, K.G. Remarkably rapid cleavage of a model phosphodiester by complexed ceric ions in aqueous micellar solutions. J. Am. Chem. Soc. 1997, 119, 9323–9324. [Google Scholar] [CrossRef]
- Calderón, A.; Yatsimirsky, A.K. Formation and phosphodiesterolytic activity of lanthanide(III) N,N-bis(2-hydroxyethyl)glycine hydroxo complexes. Inorg. Chim. Acta 2004, 357, 3483–3492. [Google Scholar] [CrossRef]
- Peluffo, E.E.; Torres, J.; Kremer, C.; Domínguez, S.; Mederos, A.; Kremer, E. Phosphodiesterolytic activity of samarium(III) mixed ligand complexes containing crown ethers and α-amino acids. Inorg. Chim. Acta 2006, 359, 2107–2114. [Google Scholar] [CrossRef]
- Torres, J.; Brusoni, M.; Peluffo, F.; Kremer, C.; Domínguez, S.; Mederos, A.; Kremer, E. Phosphodiesterolytic activity of lanthanide(III) complexes with α-amino acids. Inorg. Chim. Acta 2005, 358, 3320–3328. [Google Scholar] [CrossRef]
- Luedtke, N.W.; Schepartz, A. Lanthanide-mediated phosphoester hydrolysis and phosphate elimination from phosphopeptides. Chem. Commun. 2005, 5426–5428. [Google Scholar] [CrossRef]
- Yohannes, P.G.; Plute, K.E.; Mertes, M.P.; Mertes, K.B. Specificity, catalysis, and regulation: Effects of metal ions on polyammonium macrocycle catalyzed dephosphorylation of ATP. Inorg. Chem. 1987, 26, 1751–1755. [Google Scholar] [CrossRef]
- Schneider, H.J.; Rammo, J.; Hettich, R. Catalysis of the hydrolysis of phosphoric acid diesters by lanthanide ions and the influence of ligands. Angew. Chem. Int. Ed. 1993, 32, 1716–1719. [Google Scholar] [CrossRef]
- Breslow, R.; Zhang, B. Cleavage of phosphate esters by a cyclodextrin dimer catalyst that binds the substrates together with La3+ and hydrogen peroxide. J. Am. Chem. Soc. 1994, 116, 7893–7894. [Google Scholar] [CrossRef]
- Amin, S.; Voss, D.A.; Horrocks, D.W.; Lake, C.H.; Churchill, M.R.; Morrow, J.R. Laser-induced luminescence studies and crystal structure of the europium(III) complex of 1,4,7,10-tetrakis(carbamoylmethyl)-1,4,7,10-tetraazacyclododecane. The link between phosphate diester binding and catalysis by lanthanide(III) macrocyclic complexes. Inorg. Chem. 1995, 34, 3294–3300. [Google Scholar] [CrossRef]
- Hall, J.; Hüsken, D.; Piebes, U.; Moser, H.E.; Häner, R. Efficient sequence-specific cleavage of RNA using novel europium complexes conjugated to oligonucleotides. Chem.Biol. 1994, 1, 185–190. [Google Scholar] [CrossRef]
- Baker, B.F.; Khalili, H.; Wei, N.; Morrow, J.R. Cleavage of the 5' cap structure of mRNA by a europium(III) macrocyclic complex with pendant alcohol groups. J. Am. Chem. Soc. 1997, 119, 8749–8755. [Google Scholar] [CrossRef]
- Pratviel, G.; Bernardou, J.; Meunier, B. DNA and RNA cleavage by metal complexes. Adv. Inorg. Chem. 1998, 45, 251–312. [Google Scholar] [CrossRef]
- Komiyama, M.; Takeda, N.; Shigekawa, H. Hydrolysis of DNA and RNA by lanthanide ions: Mechanistic studies leading to new applications. Chem. Commun. 1999, 16, 1443–1451. [Google Scholar] [CrossRef]
- Bashkin, J.K. Hydrolysis of phosphates, esters and related substrates by models of biological catalysts. Curr. Opin. Chem. Biol. 1999, 3, 752–758. [Google Scholar] [CrossRef]
- Gryaznov, S.M.; Letsinger, R.L. Chemical ligation of oligonucleotides in the presence and absence of a template. J. Am. Chem. Soc. 1993, 115, 3808–3809. [Google Scholar] [CrossRef]
- Fields, G.B.; Luaer-Fields, J.L.; Liu, R.Q.; Barany, G. Synthetic Peptides—A User’s Guide; Grant, G.A., Ed.; W. H. Freeman: New York, NY, USA, 2002; pp. 93–219. [Google Scholar]
- Wagner, M.; Kunz, H. The (2-phenyl-2-trimethylsilyl)ethyl(PTMSEL) linker-A novel linker for the solid-phase synthesis of protected peptides and glycopeptides cleavable with fluoride. Angew. Chem. Int. Ed. 2002, 41, 317–321. [Google Scholar] [CrossRef]
- Lack, O.; Zbinden, H.; Woggon, W.D. A useful disulfide linker for single-bead analysis of peptide libraries. Helv. Chim. Acta 2002, 85, 495–501. [Google Scholar] [CrossRef]
- Pastor, J.J.; Fernandez, I.; Rabanal, F.; Giralt, E. A new method for the preparation of unprotected peptides on biocompatible resins with application in combinatorial chemistry. Org. Lett. 2002, 4, 3831–3833. [Google Scholar] [CrossRef]
- Bochet, C.G. Photolabile protecting groups and linkers. J. Chem. Soc. Perkin Trans. 2002, 1, 125–142. [Google Scholar]
- Shukla, D.; Tyagi, V. Synthesis and properties of geminis with two alkyl and two phosphate headgroups. Eur. J. Lipid Sci. 2008, 110, 576–580. [Google Scholar] [CrossRef]
- Ohtomo, R.; Sekiguchi, Y.; Mimura, T.; Saito, M.; Ezawa, T. Quantification of polyphosphate: Different sensitivities to short-chain polyphosphate using enzymatic and colorimetric methods as revealed by ion chromatography. Anal. Biochem. 2004, 328, 139–146. [Google Scholar] [CrossRef]
- Roigk, A.; Hettich, R.; Schneider, H.J. Unusual catalyst concentration effects in the hydrolysis of phenyl phosphate esters and of DNA: A systematic investigation of the lanthanide series. Inorg. Chem. 1998, 37, 751–756. [Google Scholar] [CrossRef]
- Chin, J. Developing artificial hydrolytic metalloenzymes by a unified mechanistic approach. Acc. Chem. Res. 1991, 24, 145–152. [Google Scholar] [CrossRef]
- Kirby, A.J. Enzyme mimics. Angew. Chem. Int. Ed. 1994, 33, 551–553. [Google Scholar] [CrossRef]
- Bunzli, J.G.; Piquet, C. Lanthanide-containing molecular and supramolecular polymetallic functional assemblies. Chem. Rev. 2002, 102, 1897–1928. [Google Scholar] [CrossRef]
- Rohwer, H.; Collier, N.; Hosten, E. Spectrophotometric study of arsenazo III and its interactions with lanthanides. Anal. Chim. Acta 1995, 314, 219–223. [Google Scholar] [CrossRef]
- Moore, F.L. Improved extraction method for isolation of trivalent actinide-lanthanide elements from nitrate solutions. Anal. Chem. 1966, 38, 510–512. [Google Scholar] [CrossRef]
- Yamada, E.; Freiser, H. Mixed ligand chelate extraction of lanthanide ions in systems involving 7-(1-vinyl-3,3,6,6-tetramethylhexyl)-8-quinolinol, 8-quinolinol, and 1,10-phenanthroline. Anal. Chem. 1981, 53, 2115–2117. [Google Scholar] [CrossRef]
- Campbell, D.O.; Buxton, S.R. Rapid ion exchange separations. Chromatographic lanthanide separations using a high-pressure ion exchange method. Ind. Eng. Chem. Proc. Des. Dev. 1970, 9, 89–94. [Google Scholar]
- Filer, T.D. Separation of the trivalent actinides from the lanthanides by extraction chromatography. Anal. Chem. 1974, 46, 608–610. [Google Scholar] [CrossRef]
- Mancin, F.; Tecilla, P. Zinc(II) complexes as hydrolytic catalysts of phosphate diester cleavage: From model substrates to nucleic acids. New J. Chem. 2007, 31, 800–817. [Google Scholar] [CrossRef]
- Letourneur, D.; Douzon, C.; Jozefowicz, M.J. Synthesis and characterization of phosphorylated polystyrene derivatives for use in chromatography: DNA-like and phospholipid-like behavior. Polym. Sci. A Polym. Chem. 1991, 29, 1367–1377. [Google Scholar] [CrossRef]
- Imbert, E.; Souirti, A.; Menard, V.; Pfluger, F.; Jozefowicz, M.; Migonney, V.J. DNA-like and phospholipid-like phosphorylated polystyrenes: Characterization, distribution of functional groups, and calcium complexation properties. Appl. Polym. Sci. 1994, 52, 91–97. [Google Scholar] [CrossRef]
- Dueymes, C.; Pirat, C.; Pascal, R. Facile synthesis of simple mono-alkyl phosphates from phosphoric acid and alcohols. Tetrahedron Lett. 2008, 49, 5300–5301. [Google Scholar] [CrossRef]
- Kaiser, E.; Colescott, R.L.; Bossinger, C.D.; Cook, P.I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal. Biochem. 1970, 34, 595–598. [Google Scholar] [CrossRef]
- Sarin, V.K.; Kent, S.B.; Tam, J.P.; Merrifield, R.B. Quantitative monitoring of solid-phase peptide synthesis by the ninhydrin reaction. Anal. Biochem. 1981, 117, 147–157. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yoo, B.; Pagel, M.D. Lanthanide-Mediated Dephosphorylation Used for Peptide Cleavage during Solid Phase Peptide Synthesis. Molecules 2013, 18, 3894-3905. https://doi.org/10.3390/molecules18043894
Yoo B, Pagel MD. Lanthanide-Mediated Dephosphorylation Used for Peptide Cleavage during Solid Phase Peptide Synthesis. Molecules. 2013; 18(4):3894-3905. https://doi.org/10.3390/molecules18043894
Chicago/Turabian StyleYoo, Byunghee, and Mark D. Pagel. 2013. "Lanthanide-Mediated Dephosphorylation Used for Peptide Cleavage during Solid Phase Peptide Synthesis" Molecules 18, no. 4: 3894-3905. https://doi.org/10.3390/molecules18043894




