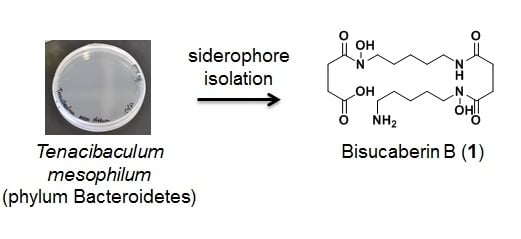
Bisucaberin B, a Linear Hydroxamate Class Siderophore from the Marine Bacterium Tenacibaculum mesophilum
Abstract
:1. Introduction
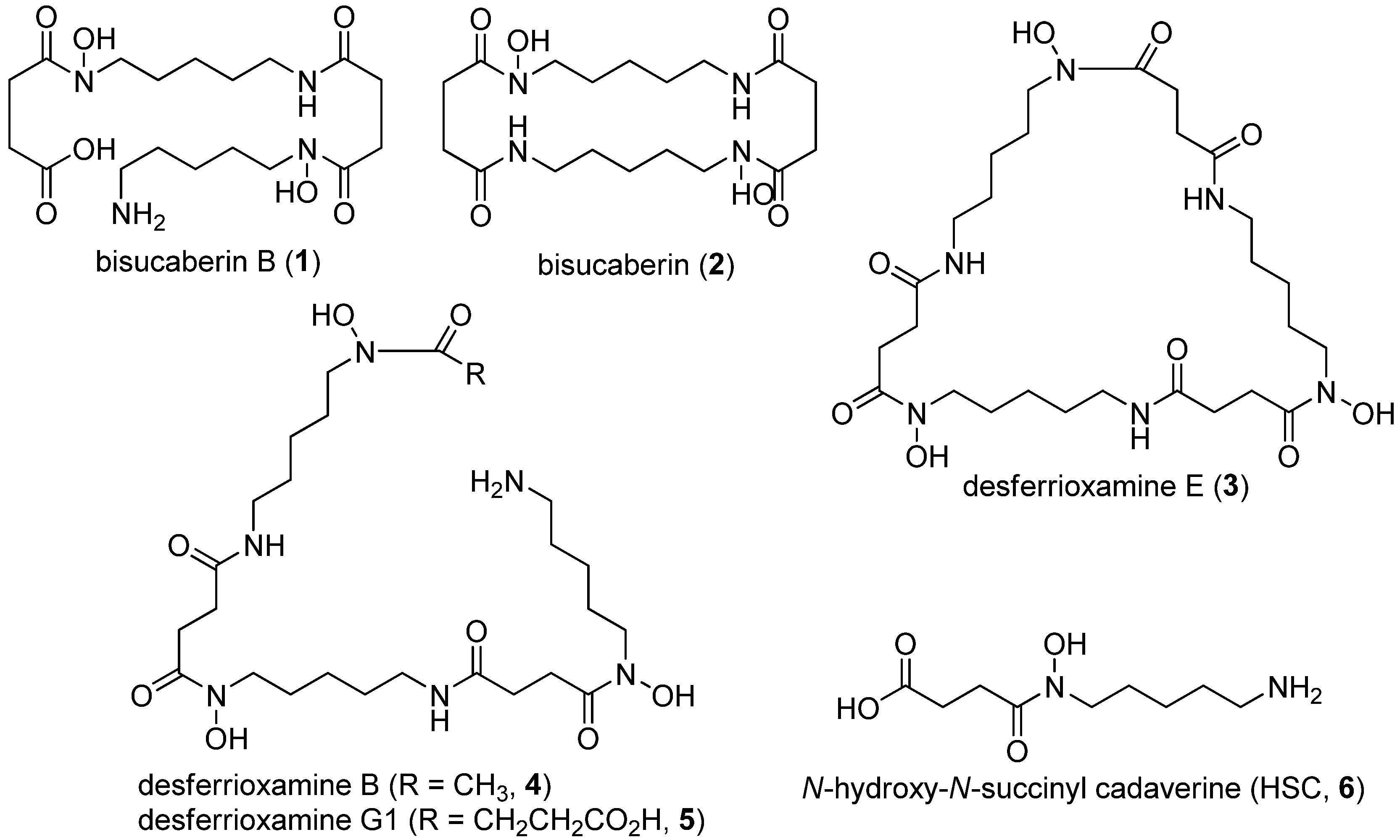
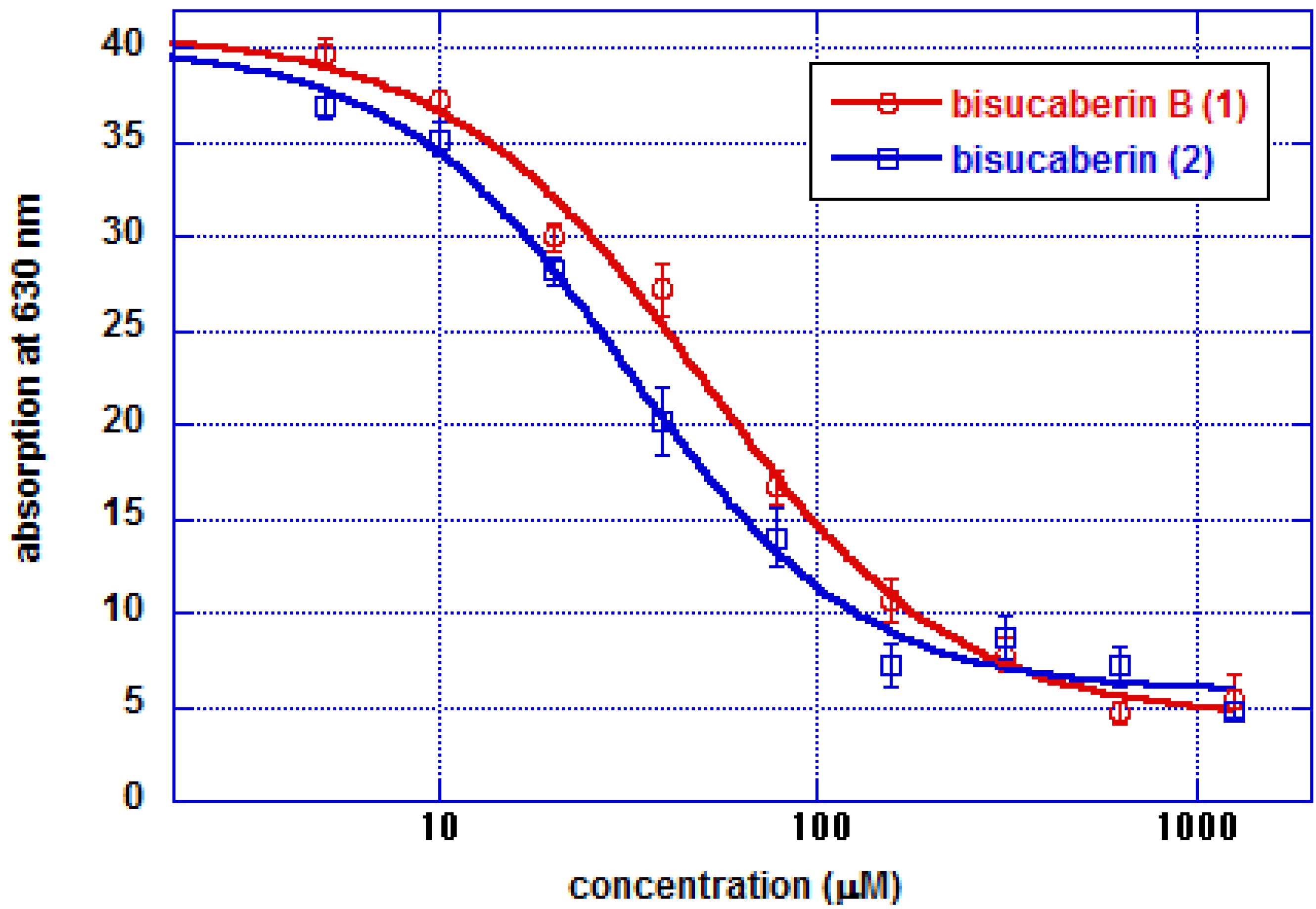
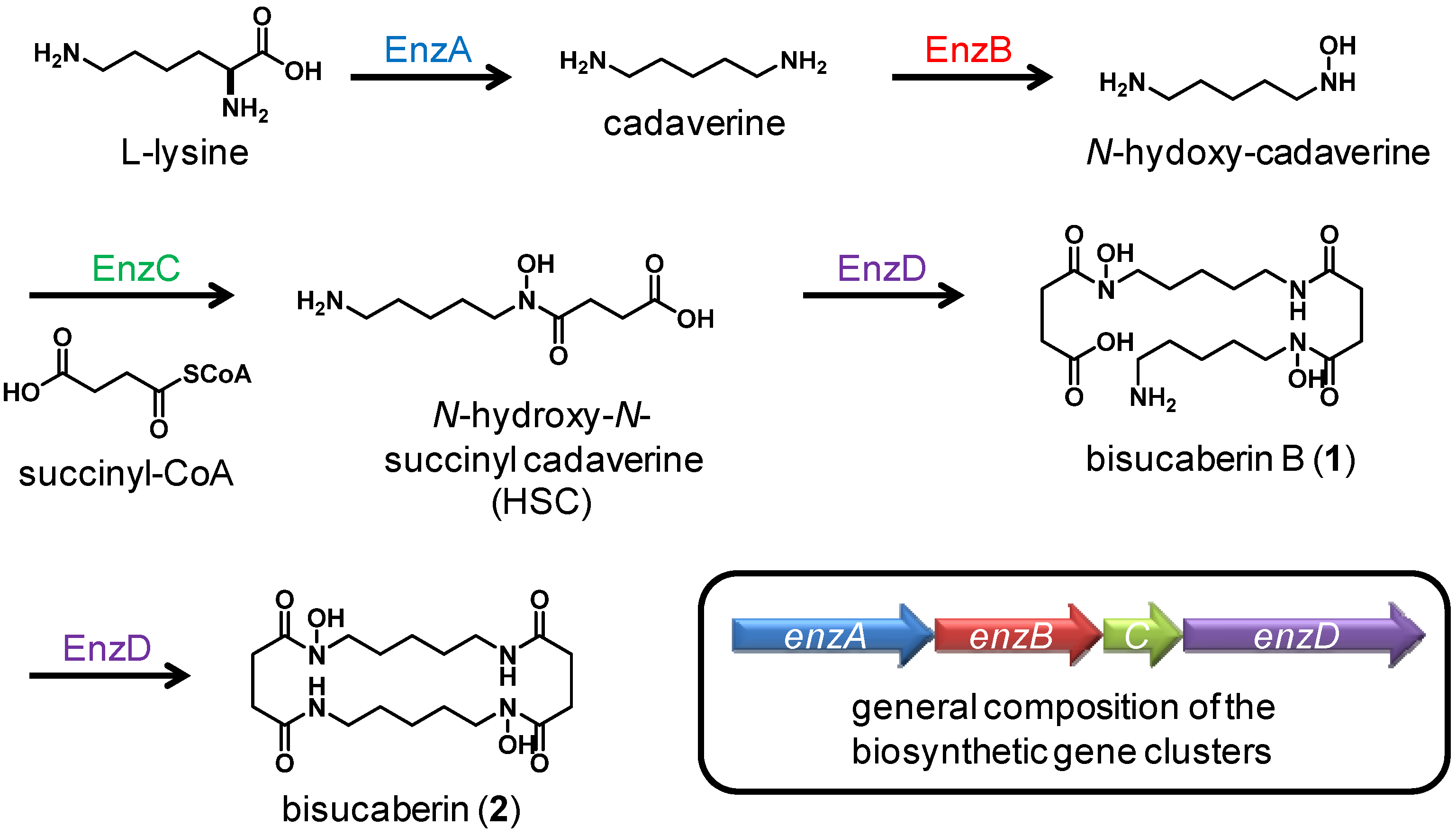
2. Results and Discussion
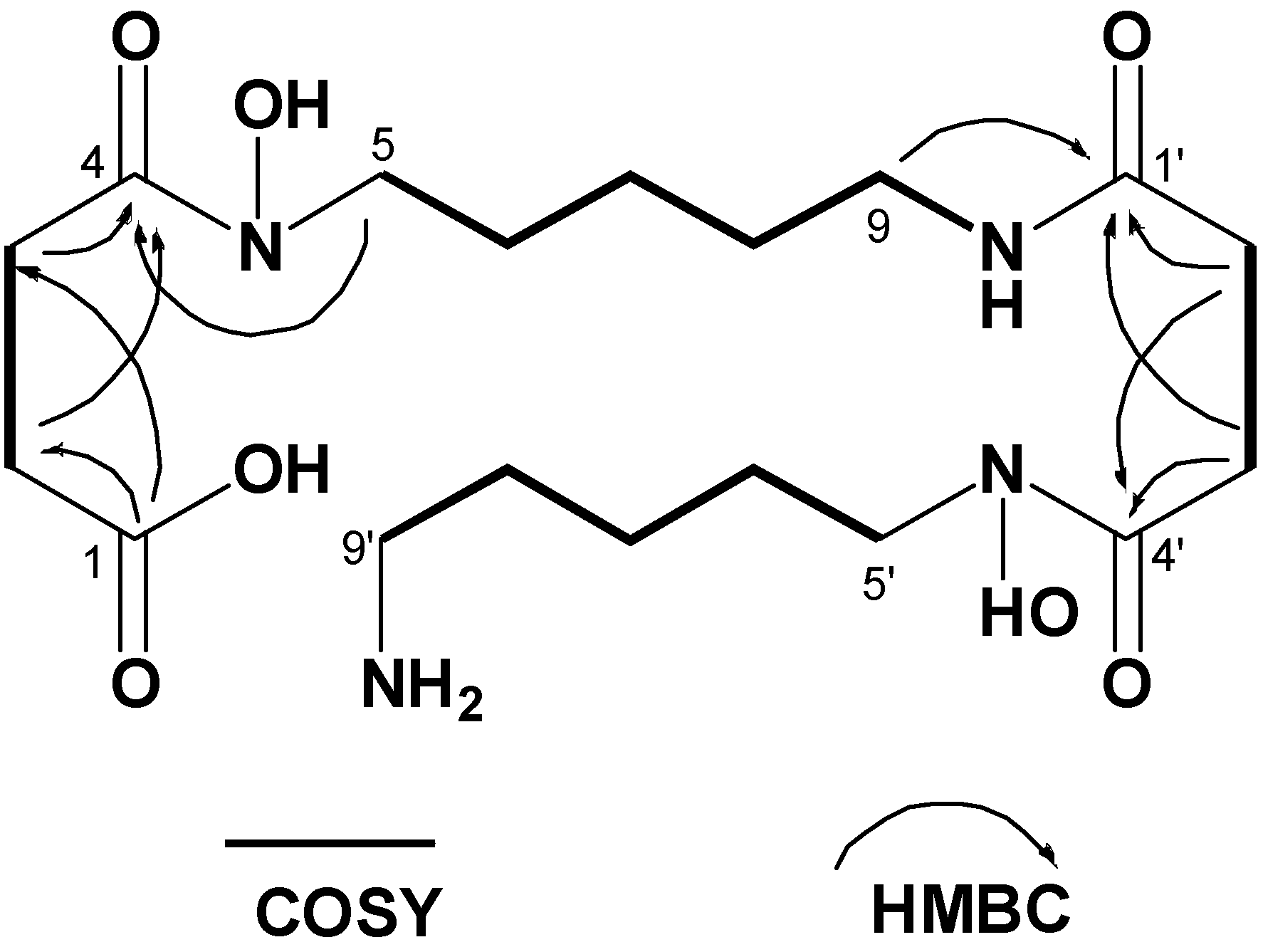
| δH mult. (J (Hz)) | δC | δH mult. (J (Hz)) | δC | ||
|---|---|---|---|---|---|
| 1 | 175.8 | 1' | 171.5 | ||
| 2 | 2.44 t (6.4) | 28.0 | 2' | 2.29 t (6.4) | 27.8 |
| 3 | 2.31 t (6.4) | 33.0 | 3' | 2.59 t (6.4) | 30.3 |
| 4 | 171.9 | 4' | 171.9 | ||
| 5 | 3.45 t (6.4) | 46.1 | 5' | 3.48 t (6.4) | 46.6 |
| 6 | 1.47a | 25.8 | 6' | 1.51 a | 25.5 |
| 7 | 1.21 quint. (7.2) | 23.1 | 7' | 1.27 quint. (6.8) | 22.7 |
| 8 | 1.35 quint. (6.8) | 28.6 | 8' | 1.49 a | 27.2 |
| 9 | 2.99 quint. (6.4) | 38.1 | 9' | 2.71 t (6.4) | 38.6 |
| NH | 7.75 t (6.4) |
3. Experimental
3.1. General Procedures
3.2. Bacterial Strain
3.3. Culture and Isolation
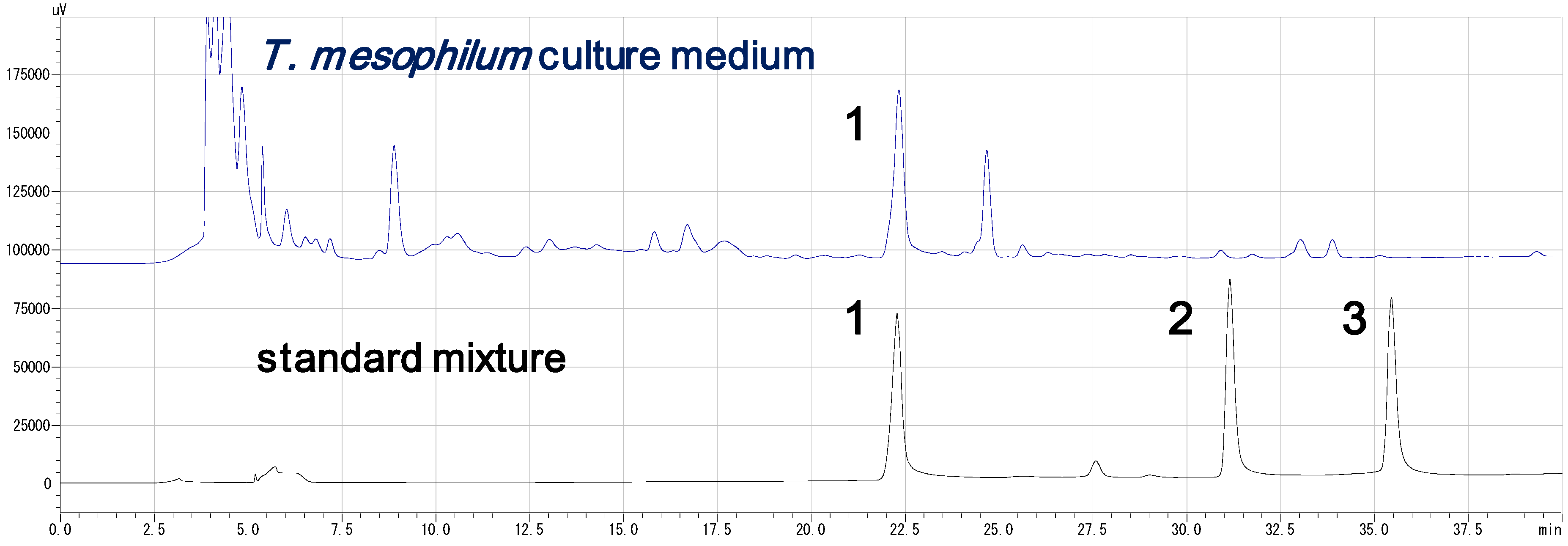
3.4. HPLC Analysis of the Standard Compounds and Cultured Medium (Figure 2)
3.5. Methylation
3.6. CAS Plate Assay
3.7. CAS Solution Assay
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
References
- Sandy, M.; Butler, A. Microbial iron acquisition: Marine and terrestrial siderophores. Chem. Rev. 2009, 109, 4580–4595. [Google Scholar] [CrossRef]
- Luckey, M.; Pollack, J.R.; Wayne, R.; Ames, B.N.; Neilands, J.B. Iron uptake in salmonella typhimurium. Utilization of exogenous siderochromes as iron carriers. J. Bacteriol. 1972, 111, 731–738. [Google Scholar]
- D'Onofrio, A.; Crawford, J.M.; Stewart, E.J.; Witt, K.; Gavrish, E.; Epstein, S.; Clardy, J.; Lewis, K. Siderophores from neighboring organisms promote the growth of uncultured bacteria. Chem. Biol. 2010, 17, 254–264. [Google Scholar] [CrossRef]
- Seyedsayamdost, M.R.; Cleto, S.; Carr, G.; Vlamakis, H.; Joao Vieira, M.; Kolter, R.; Clardy, J. Mixing and matching siderophore clusters: Structure and biosynthesis of serratiochelins from serratia sp. V4. J. Am. Chem. Soc. 2012, 134, 13550–13553. [Google Scholar]
- Traxler, M.F.; Seyedsayamdost, M.R.; Clardy, J.; Kolter, R. Interspecies modulation of bacterial development through iron competition and siderophore piracy. Mol. Microbiol. 2012, 86, 628–644. [Google Scholar] [CrossRef]
- Crosa, J.H.; Walsh, C.T. Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 223–249. [Google Scholar] [CrossRef]
- Kadi, N.; Song, L.; Challis, G.L. Bisucaberin biosynthesis: An adenylating domain of the bibc multi-enzyme catalyzes cyclodimerization of n-hydroxy-n-succinylcadaverine. Chem. Commun. 2008, 41, 5119–5121. [Google Scholar] [CrossRef]
- Barona-Gomez, F.; Wong, U.; Giannakopulos, A.E.; Derrick, P.J.; Challis, G.L. Identification of a cluster of genes that directs desferrioxamine biosynthesis in streptomyces coelicolor m145. J. Am. Chem. Soc. 2004, 126, 16282–16283. [Google Scholar] [CrossRef]
- Kadi, N.; Oves-Costales, D.; Barona-Gomez, F.; Challis, G.L. A new family of atp-dependent oligomerization-macrocyclization biocatalysts. Nat. Chem. Biol. 2007, 3, 652–656. [Google Scholar] [CrossRef]
- Fujita, M.J.; Kimura, N.; Yokose, H.; Otsuka, M. Heterologous production of bisucaberin using a biosynthetic gene cluster cloned from a deep sea metagenome. Mol. Biosyst. 2012, 8, 482–485. [Google Scholar] [CrossRef]
- Winkelmann, G.; Busch, B.; Hartmann, A.; Kirchhof, G.; Sussmuth, R.; Jung, G. Degradation of desferrioxamines by azospirillum irakense: Assignment of metabolites by hplc/electrospray mass spectrometry. BioMetals 1999, 12, 255–264. [Google Scholar]
- Suzuki, M.; Nakagawa, Y.; Harayama, S.; Yamamoto, S. Phylogenetic analysis and taxonomic study of marine cytophaga-like bacteria: Proposal for tenacibaculum gen. Nov. With tenacibaculum maritimum comb. Nov. And tenacibaculum ovolyticum comb. Nov., and description of tenacibaculum mesophilum sp. Nov. And tenacibaculum amylolyticum sp. Nov. Int. J. Syst. Evol. Microbiol 2001, 51, 1639–1652. [Google Scholar]
- Kameyama, T.; Takahashi, A.; Kurasawa, S.; Ishizuka, M.; Okami, Y.; Takeuchi, T.; Umezawa, H. Bisucaberin, A new siderophore, Sensitizing tumor cells to macrophage-mediated cytolysis. I. Taxonomy of the producing organism, Isolation and biological properties. J. Antibiot. 1987, 40, 1664–1670. [Google Scholar] [CrossRef]
- Winkelmann, G.; Schmid, D.G.; Nicholson, G.; Jung, G.; Colquhoun, D.J. Bisucaberin-a dihydroxamate siderophore isolated from vibrio salmonicida, An important pathogen of farmed atlantic salmon (salmo salar). BioMetals 2002, 15, 153–160. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Imbert, M.; Bechet, M.; Blondeau, R. Comparison of the main siderophores produced by some species of streptomyces. Curr. Microbiol. 1995, 31, 129–133. [Google Scholar] [CrossRef]
- Zawadzka, A.M.; Vandecasteele, F.P.J.; Crawford, R.L.; Paszczynski, A.J. Identification of siderophores of pseudomonas stutzeri. Can. J. Microbiol. 2006, 52, 1164–1176. [Google Scholar] [CrossRef]
- Feistner, G.J.; Ishimaru, C. Proferrioxamine profiles of erwinia herbicola and related bacteria. BioMetals. 1996, 9, 337–344. [Google Scholar]
- Martinez, J.S.; Haygood, M.G.; Butler, A. Identification of a natural desferrioxamine siderophore produced by a marine bacterium. Limnol. Oceanogr. 2001, 46, 420–424. [Google Scholar] [CrossRef]
- Eden, P.A.; Schmidt, T.M.; Blakemore, R.P.; Pace, N.R. Phylogenetic analysis of aquaspirillum magnetotacticum using polymerase chain reaction-amplified 16s rrna-specific dna. Int. J. Syst. Bacteriol. 1991, 41, 324–325. [Google Scholar] [CrossRef]
- Tsukimoto, M.; Nagaoka, M.; Shishido, Y.; Fujimoto, J.; Nishisaka, F.; Matsumoto, S.; Harunari, E.; Imada, C.; Matsuzaki, T. Bacterial production of the tunicate-derived antitumor cyclic depsipeptide didemnin b. J. Nat. Prod. 2011, 74, 2329–2331. [Google Scholar]
- Sample Availability: Samples of the compounds bisucaberin B (1), bisucaberin (2), and desferrioxamine E (3) are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fujita, M.J.; Nakano, K.; Sakai, R. Bisucaberin B, a Linear Hydroxamate Class Siderophore from the Marine Bacterium Tenacibaculum mesophilum. Molecules 2013, 18, 3917-3926. https://doi.org/10.3390/molecules18043917
Fujita MJ, Nakano K, Sakai R. Bisucaberin B, a Linear Hydroxamate Class Siderophore from the Marine Bacterium Tenacibaculum mesophilum. Molecules. 2013; 18(4):3917-3926. https://doi.org/10.3390/molecules18043917
Chicago/Turabian StyleFujita, Masaki J., Koji Nakano, and Ryuichi Sakai. 2013. "Bisucaberin B, a Linear Hydroxamate Class Siderophore from the Marine Bacterium Tenacibaculum mesophilum" Molecules 18, no. 4: 3917-3926. https://doi.org/10.3390/molecules18043917