Volatile and Amino Acid Profiling of Dry Cured Hams from Different Swine Breeds and Processing Methods
Abstract
:1. Introduction
2. Results and Discussion
2.1. Amino Acids and Related Compounds
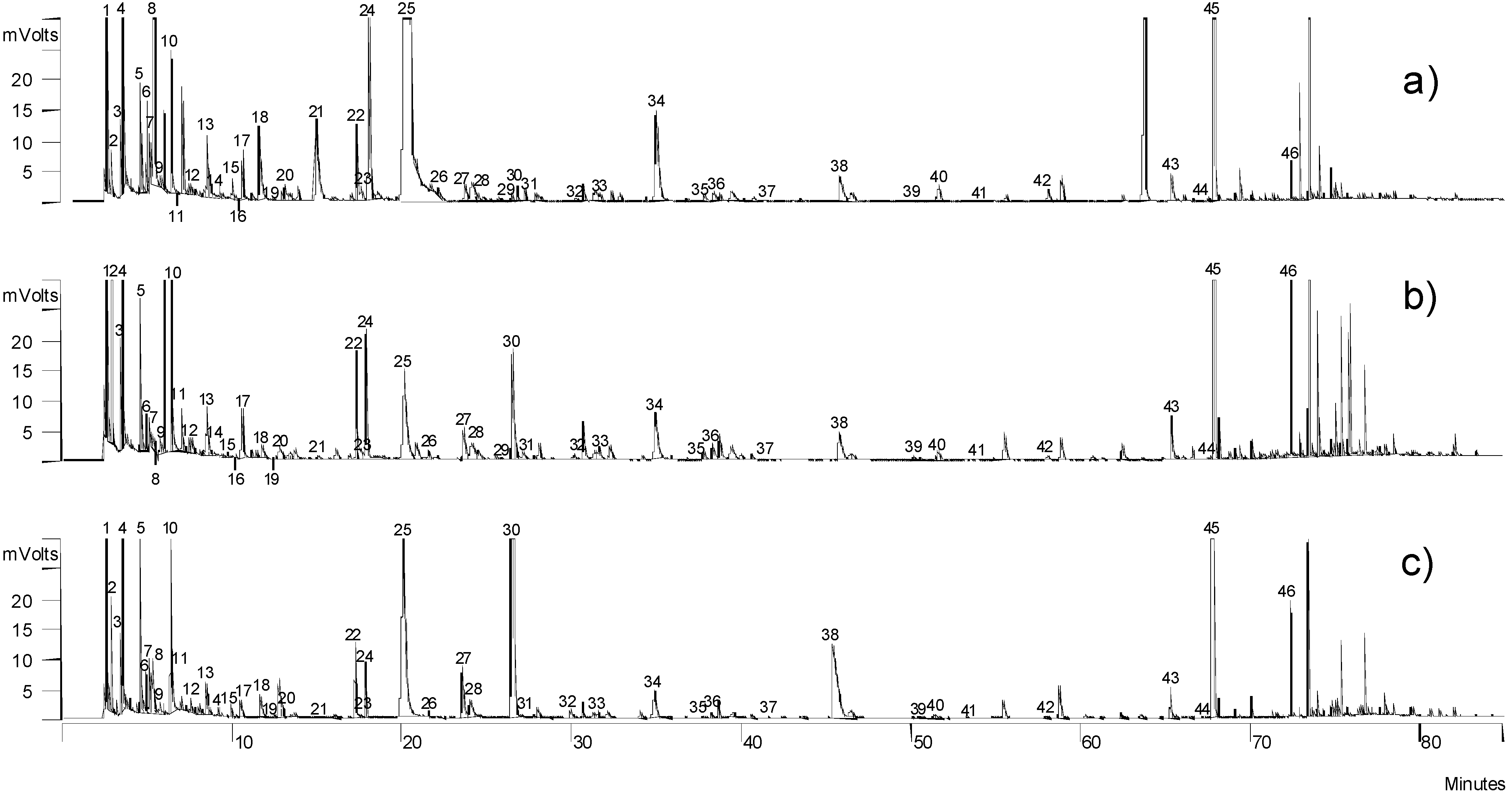
2.2. Volatile Compounds
| Code | Compound | Non Iberian a | Iberian a | p |
|---|---|---|---|---|
| A1 | Tryptophan | 23 ± 1 | 21 ± 1 | 0.30 |
| A2 | Phenyalanine | 252 ± 55 | 286 ± 67 | <0.01 |
| A3 | Tyrosine | 103 ± 3 | 150 ± 11 | <0.01 |
| A4 | Tyramine | 12 ± 2 | 2 ± 1 | 0.02 |
| A5 | Isoleucine | 300 ± 8 | 391 ± 10 | <0.01 |
| A6 | Leucine | 267 ± 7 | 353 ± 11 | <0.01 |
| A7 | Methionine | 157 ± 1 | 209 ± 1 | <0.01 |
| A8 | Valine | 316 ± 1 | 402 ± 1 | <0.01 |
| A9 | Creatine | 1637 ± 19 | 118 ± 23 | <0.01 |
| A10 | Proline | 146 ± 4 | 197 ± 6 | <0.01 |
| A11 | Creatinine | 29 ± 1 | 36 ± 1 | <0.01 |
| A12 | Glutamic acid | 543 ± 162 | 739 ± 25 | <0.01 |
| A13 | Arginine | 195 ± 6 | 301 ± 9 | <0.01 |
| A14 | Asparagine | 185 ± 50 | 195 ± 15 | 0.50 |
| A15 | Taurine | 57 ± 1 | 86 ± 8 | <0.01 |
| A16 | Histidine | 165 ± 5 | 226 ± 6 | <0.01 |
| A17 | Serine | 181 ± 7 | 255 ± 5 | <0.01 |
| A18 | Glycine | 92 ± 3 | 119 ± 2 | <0.01 |
| A19 | Lysine | 686 ± 22 | 966 ± 29 | <0.01 |
| Code | Rt | Volatile compound | Non Iberian a | Iberian a | p | OT | GC-O |
|---|---|---|---|---|---|---|---|
| V1 | 0.16 | Hexane | 0.36 ± 0.03 | 0.29 ± 0.03 | 0.20 | 1.50 | Spicy |
| V2 | 0.17 | Heptane | 0.22 ± 0.03 | 0.23 ± 0.03 | 0.85 | 0.67 | Sweety, alkane |
| V3 | 0.20 | Octane | 2.23 ± 0.42 | 3.00 ± 0.41 | 0.35 | 0.94 | Sweety, alkane |
| V4 | 0.21 | 2-Propanone | 1.72 ± 0.13 | 2.21 ± 0.32 | 0.10 | 500 | Fruity, apple, cooked meat |
| V5 | 0.27 | 2-Butanone | 0.34 ± 0.04 | 0.20 ± 0.02 | 0.03 | 40 | Ethereal |
| V6 | 0.29 | 3-Methylbutanal | 0.13 ± 0.02 | 0.38 ± 0.06 | <0.01 | 0.08 | Acorn, fruity, cheesy, salty |
| V7 | 0.31 | 2-Propanol | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.95 | 26 | Alcoholic, dry, buttery-taste |
| V8 | 0.32 | Ethanol | 1.31 ± 0.20 | 1.52 ± 0.26 | 0.61 | 30 | Alcohol, sweet |
| V9 | 0.34 | 2-Ethylfuran | 0.09 ± 0.04 | 0.07 ± 0.01 | 0.74 | - | Sweet |
| V10 | 0.38 | 2-Pentanone + 3-Pentanone | 0.79 ± 0.08 | 0.48 ± 0.10 | 0.05 | 70 b | Sweet, fruity, green |
| V11 | 0.39 | 2,3-Butanedione | 0.36 ± 0.07 | 0.35 ± 0.10 | 0.95 | - | Vanilla/caramel-like, buttery |
| V12 | 0.46 | α-Pinene | 0.09 ± 0.01 | 0.08 ± 0.02 | 0.57 | 0.02 | Sharp, pine |
| V13 | 0.51 | Methyl benzene | 0.10 ± 0.01 | 0.12 ± 0.01 | 0.03 | 0.33 | Plastic, glue, strong |
| V14 | 0.53 | 2-Methyl-3-buten-2-ol | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.74 | 0.48 | Earthy |
| V15 | 0.60 | Dimethyl disulfide | 0.02 ± 0.00 c | 0.02 ± 0.00 c | 0.39 | 0.01 | Cauliflowers, vegetable |
| V16 | 0.61 | Butyl acetate | 0.01 ± 0.00 c | 0.01 ± 0.00 c | 0.80 | 0.30 | Fruity, banana, apple |
| V17 | 0.64 | Hexanal | 1.18 ± 0.20 | 3.76 ± 0.64 | <0.01 | 0.08 | Green, grassy, fatty |
| V18 | 0.69 | 2-Methyl propanol | 0.11 ± 0.01 | 0.32 ± 0.04 | <0.01 | 1.00 | Wine, penetrating |
| V19 | 0.75 | 2-Butanol | 0.03 ± 0.01 | 0.02 ± 0.00 | 0.16 | 0.50 | Winey |
| V20 | 0.78 | Ethyl benzene | 0.03 ± 0.00 c | 0.02 ± 0.00 c | 0.81 | - | Dry, glue, unpleasant |
| V21 | 0.90 | Butanol | 0.04 ± 0.02 | 0.15 ± 0.01 | 0.01 | 0.04 | Fruity, medicinal |
| V22 | 1.05 | 2-Heptanone | 1.56 ± 0.17 | 1.24 ± 0.28 | 0.36 | 0.30 | Spicy, acorn, blue cheese |
| V23 | 1.06 | Heptanal | 1.03 ± 0.33 | 1.47 ± 0.21 | 0.49 | 0.50 | Fatty, greasy, ham-like |
| V24 | 1.09 | Limonene | 0.59 ± 0.10 | 2.68 ± 0.56 | <0.01 | 0.25 | Citric, fresh |
| V25 | 1.21 | 3-Methylbutanol | 1.33 ± 0.11 | 5.27 ± 1.15 | <0.01 | 0.10 | Woody, acorn, pleasant green |
| V26 | 1.31 | 2-Pentylfuran | 0.49 ± 0.08 | 0.94 ± 0.22 | 0.01 | 0.10 | Green fruity, butter |
| V27 | 1.43 | Octan-3-one | 1.25 ± 0.07 | 0.63 ± 0.08 | <0.01 | 0.01 | Spicy, mushroom, dirty |
| V28 | 1.46 | Pentanol | 1.25 ± 0.15 | 1.26 ± 0.06 | 0.99 | 0.47 | Pungent, strong, balsamic |
| V29 | 1.59 | (E,E)-2,4-Decadienal | 0.46 ± 0.06 | 0.03 ± 0.01 | <0.01 | 2.50 | Fatty, rancid |
| V30 | 1.61 | 2-Octanone | 2.20 ± 0.37 | 0.72 ± 0.12 | 0.03 | 0.51 | Fruity, floral, green, fresh |
| V31 | 1.63 | Octanal | 4.28 ± 0.88 | 7.38 ± 1.10 | 0.04 | 0.32 | Meat-like, green, fresh |
| V32 | 1.84 | (E)-2-Heptenal | 1.33 ± 0.33 | 0.71 ± 0.19 | 0.31 | 0.05 | Green, fatty, fruity, almonds |
| V33 | 1.89 | 2-Heptanol | 0.55 ± 0.06 | 0.70 ± 0.13 | 0.27 | 0.01 | Oily, sweety |
| V34 | 2.09 | Hexanol | 1.74 ± 0.18 | 4.10 ± 0.59 | <0.01 | 0.40 | Fruity, green |
| V35 | 2.30 | 2-Nonanone | 1.13 ± 0.22 | 1.69 ± 0.52 | 0.26 | 0.10 | Floral, fruity, blue cheese |
| V36 | 2.33 | Nonanal | 2.50 ± 0.44 | 4.33 ± 0.67 | 0.04 | 0.15 | Rancid, fatty, tallowy |
| V37 | 2.55 | (E)-2-Octenal | 0.84 ± 0.16 | 2.47 ± 0.58 | <0.01 | 0.00 d | Leaves, pungent, fatty, fruity |
| V38 | 2.76 | 1-Octen-3-ol | 2.72 ± 0.20 | 1.66 ± 0.21 | 0.01 | 0.00 d | Mushroom-like, earthy, dust |
| V39 | 3.02 | Decanal | 0.26 ± 0.02 | 0.28 ± 0.03 | 0.74 | 0.65 | Citrus, waxy |
| V40 | 3.11 | Benzaldehyde | 0.97 ± 0.09 | 1.78 ± 0.21 | <0.01 | 0.06 | Bitter almonds, penetrating |
| V41 | 3.22 | (E)-2-Nonenal | 2.03 ± 0.37 | 3.88 ± 0.92 | 0.03 | 0.15 | Fatty, waxy |
| V42 | 3.47 | Octanol | 0.42 ± 0.05 | 0.98 ± 0.12 | <0.01 | 0.03 | Fatty, sharp |
| V43 | 3.94 | Butanoic acid | 0.57 ± 0.05 | 0.61 ± 0.09 | 0.69 | 0.65 | Cheesy, rancid |
| V44 | 4.13 | Nonanol | 0.19 ± 0.01 | 0.26 ± 0.04 | 0.08 | 0.28 | Fatty green |
| V45 | 4.14 | Isobutyric acid | 6.49 ± 0.46 | 4.99 ± 0.67 | 0.12 | 8.10 | Iron, fishy |
| V46 | 4.35 | Hexanoic acid | 4.76 ± 0.58 | 4.90 ± 0.88 | 0.12 | 0.70 | Fatty, cheese, sweaty |
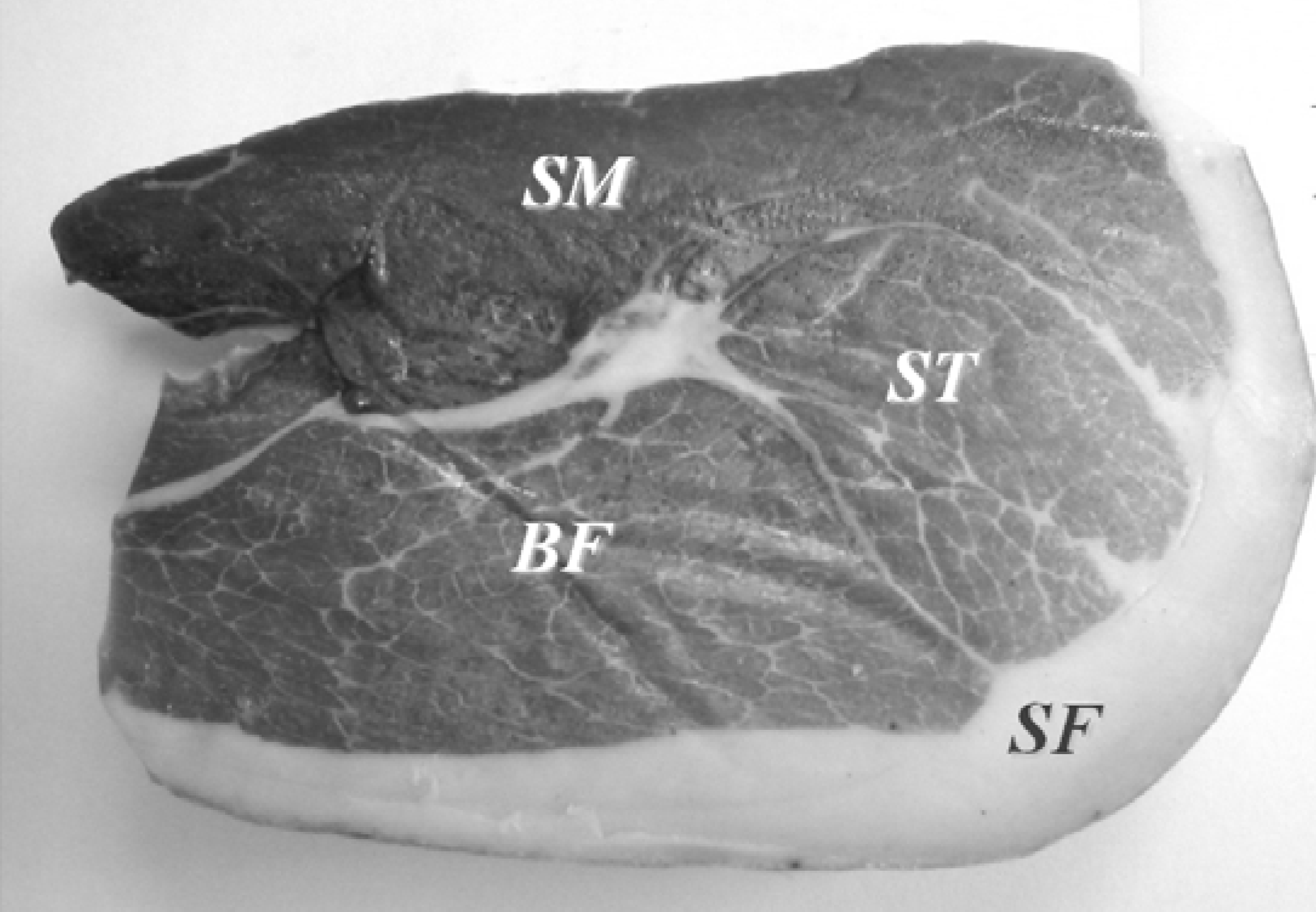
| Chemical series | Volatile compounds | BF a | SF a | SM a | ST a |
|---|---|---|---|---|---|
| Hydrocarbons | Hexane | 0.40 (0.03–1.77) | 0.27 (0.04–0.66) | 0.37 (0.03–0.97) | 0.38 (0.04–1.25) |
| Heptane | 0.16 (0.03–1.13) | 0.26 (0.05–0.70) | 0.29 (0.03–2.99) | 0.15 (0.02–0.58) | |
| Octane | 1.66 (0.10–7.91) | 3.22 (0.52–10.81) | 2.12 (0.39–5.30) | 1.37 (0.40–3.81) | |
| Methyl benzene | 0.14 (0.04–0.37) | 0.15 (0.02–0.44) | 0.19 (0.07–0.42) | 0.13 (0.06–0.24) | |
| Ethyl benzene | 0.12 (tr-0.35) | 0.20 (tr-0.73) | 0.17 (0.01–0.74) | 0.14 (0.01–0.71) | |
| Limonene | 0.40 (0.01–4.71) | 0.51 (tr-3.16) | 1.42 (tr-14.60) | 0.56 (tr-5.14) | |
| α-Pinene | 0.19 (tr-1.61) | 0.10 (tr-0.82) | 0.28 (tr-1.38) | 0.22 (0.02–1.56) | |
| Alcohols | 2-Propanol | 0.32 (0.01–2.64) | 0.11 (tr-0.52) | 0.33 (tr-3.25) | 0.29 (tr-1.62) |
| Ethanol | 0.70 (0.01–5.86) | 0.40 (0.01–3.52) | 0.83 (0.01–8.90) | 0.79 (0.01–5.46) | |
| 2-Methyl-3-buten-2-ol | 0.04 (tr-0.24) | 0.11 (tr-0.42) | 0.04 (tr-0.19) | 0.05 (tr-0.40) | |
| 2-Methyl propanol | 0.13 (tr-0.63) | 0.08 (tr-0.26) | 0.15 (0.01–0.75) | 0.12 (tr-0.70) | |
| 2-Butanol | 0.02 (tr-0.27) | 0.03 (tr-0.13) | 0.03 (tr-0.49) | 0.03 (tr-0.40) | |
| Butanol | 0.28 (tr-8.50) | 0.05 (tr-0.16) | 0.09 (tr-0.37) | 0.09 (tr-0.79) | |
| 3-Methyl butanol | 3.10 (0.06–21.31) | 0.65 (0.04–6.13) | 1.76 (0.02–1 0.63) | 2.04 (0.02–17.99) | |
| Pentanol | 1.19 (0.07–7.66) | 1.48 (0.28–4.81) | 1.26 (0.09–7.43) | 1.17 (0.11–8.57) | |
| 2-Heptanol | 0.66 (tr-5.06) | 0.71(0.04–3.12) | 0.72 (0.09–3.76) | 0.66 (0.05–3.27) | |
| Hexanol | 1.56 (0.14–11.31) | 2.84 (0.03–10.13) | 2.10 (0.45–6.32) | 2.55 (0.34–9.77) | |
| 1-Octen-3-ol | 2.48 (tr-7.73) | 2.27 (0.07–8.40) | 0.54 (0.11–1.65) | 0.65 (0.09–2.54) | |
| Octanol | 0.56 (tr-3.02) | 0.79 (0.13–2.90) | 0.52 (tr-2.13) | 0.53 (tr-2.25) | |
| Nonanol | 0.23 (0.02–1.02) | 0.38 (0.08–0.92) | 0.19 (0.03–0.49) | 0.19 (0.02–1.09) | |
| 3-Methylbutanal | 0.20 (0.02–1.23) | 0.13 (0.01–046) | 0.40 (tr-2.23) | 0.21 (0.01–1.19) | |
| Hexanal | 0.80 (0.05–.41) | 3.29 (0.03–15.58) | 1.20 (0.08–11.40) | 0.74 (0.06–7.17) | |
| Heptanal | 0.82 (tr-6.11) | 2.51 (0.01–8.76) | 0.87 (tr-4.01) | 0.77 (tr-4.89) | |
| (E,E)-2,4-Decadienal | 0.36 (tr-2.45) | 0.25 (tr-1.56) | 0.26 (tr-2.97) | 0.43 (tr-4.80) | |
| Octanal | 1.70 (tr-8.60) | 1 0.45 (0.04–37.40) | 5.10 (0.04–28.46) | 4.11 (tr-30.95) | |
| (E)-2-Heptenal | 1.89 (0.01–50.59) | 1.68 (0.01–15.22) | 2.69 (0.04–10.67) | 1.97 (0.01–35.65) | |
| Nonanal | 4.57 (tr-67.17) | 5.05 (0.03–18.97) | 4.91 (tr-23.51) | 4.05 (tr-31.84) | |
| (E)-2-Octenal | 0.43 (0.02–3.01) | 2.23(0.03–18.61) | 0.59 (0.02–7.65) | 0.33 (0.02–3.24) | |
| Decanal | 0.17 (tr-0.82) | 0.18 (tr-0.77) | 0.20 (0.05–0.57) | 0.16 (tr-2.15) | |
| Benzaldehyde | 1.27 (0.21–5.74) | 0.86 (0.12–2.29) | 1.54 (0.01–5.42) | 1.10 (0.2–3.84) | |
| (E)-2-Nonenal | 1.14 (0.01–9.22) | 4.64 (0.14–25.14) | 1.18 (0.14–8.18) | 0.87 (0.08–7.94) | |
| 2-Octanone | 1.89 (tr-13.36) | 0.93 (tr-4.17) | 0.67 (tr-7.27) | 1.88 (tr-16.27) | |
| 2-Propanone | 2.88 (0.09–9.45) | 1.53 (0.12–6.67) | 2.73 (0.301 0.29) | 2.78 (0.03–12.47) | |
| 2-Butanone | 0.28 (0.02–1.16) | 0.13 (0.05–0.30) | 0.41 (0.01–2.34) | 0.27 (0.08–0.82) | |
| 2-Pentanone b | 0.70 (tr-2.46) | 0.57 (tr-3.54) | 0.70 (tr-3.67) | 0.80 (tr-3.82) | |
| 2,3-Butanedione | 0.47 (tr-7.51) | 0.57 (tr-3.83) | 0.41 (tr-6.84) | 0.47 (tr-4.71) | |
| 2-Heptanone | 185 (tr-7.77) | 1.53 (tr-10.14) | 2.01 (0.20–9.30) | 2.45 (tr-14.48) | |
| Octen-3-one | 0.77 (0.05–2.25) | 0.89 (0.08–2.44) | 1.07 (0.02–3.19) | 0.79 (tr-2.01) | |
| 2-Nonanone | 1.46 (0.08–1 0.73) | 2.47 (tr-11.16) | 2.17 (0.15–18.46) | 2.31 (0.20–13.75) | |
| Butanoic acid | 0.47 (0.03–1.48) | 0.64 (0.04–2.67) | 0.49 (0.06–1.78) | 0.56 (0.06–1.72) | |
| Isobutyric acid | 6.10 (0.48–20.72) | 4.45 (0.32–11.55) | 4.41 (0.50–17.58) | 5.37 (0.56–12.85) | |
| Hexanoic acid | 2.70 (0.24–18.07) | 7.60 (0.10–28.98) | 4.01 (0.25–24.37) | 4.39 (0.27–44.19) | |
| 2-Pentylfuran | 0.34 (tr-2.28) | 1.15 (0.03–5.09) | 0.63 (tr-3.09) | 0.23 (tr-1.43) | |
| 2-Ethylfuran | 0.17 (tr-5.58) | 0.11 (0.01–0.29) | 0.09 (tr-0.31) | 0.06 (tr-0.28) | |
| Butyl acetate | 0.03 (tr-0.28) | 0.01 (tr-0.04) | 0.02 (tr-0.07) | 0.02 (tr-0.07) | |
| Dimethyl disulfide | 0.03 (tr-0.22) | 0.02 (tr-0.13) | 0.05 (tr-1.10) | 0.03 (tr-0.14) |
2.2.1. Hydrocarbons
2.2.2. Alcohols
| Amino acids | Volatile compounds | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V7 | V8 | V14 | V19 | V21 | V25 | V28 | V34 | V33 | V38 | V42 | V44 | |
| Tryptophan | SF:0.75 | BF:0.79 | BF:0.80 | |||||||||
| BF:0.85 ST:0.80 SM:0.89 | ST:0.76 | ST:0.81 | ||||||||||
| ST:0.92 SF:0.79 | ||||||||||||
| BF:0.85 SF:0.79 | BF:0.82 | SF:0.89 BF:0.92 | BF:0.83 ST:0.79 SM:0.93 | BF:0.75 | BF:0.82 ST:0.87 | |||||||
| BF:0.77 | ||||||||||||
| ST:0.82 | ST:0.87 | BF:0.93 | BF:0.81 ST:0.80 | ST:0.75 | ||||||||
| BF:0.85 | ||||||||||||
2.2.3. Aldehydes
| Amino acids | Volatile compounds | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| V6 | V17 | V23 | V29 | V31 | V32 | V36 | V37 | V39 | V40 | |
| Tryptophan | ST:0.83 SM:0.84 | |||||||||
| ST:0.88 | ST:0.91 | ST:0.78 | ST:0.77 | |||||||
| BF:0.81 | SF:0.78 BF:0.78 | |||||||||
| ST:0.89 | ST:0.85 | |||||||||
| BF:0.78 | BF:0.86 | |||||||||
| SM:0.84 | BF:0.88 | |||||||||
| ST:0.87 | ST:0.79 | ST:0.80 | ||||||||
| ST:0.82 BF:0.77 | ST:0.79 | |||||||||
| BF:0.94 | BF:0.91 | BF:0.84 | BF:0.82 | BF:0.75 | ST:0.76 | |||||
| ST:0.92 | ST:0.75 | |||||||||
| ST:0.87 | ||||||||||
| ST:0.78 | ||||||||||
2.2.4. Ketones
2.2.5. Acids
2.2.6. Furans
2.2.7. Esters
2.2.8. Sulfur Compounds
2.3. Effect of Breed on Dry Cured Ham Aroma
3. Experimental
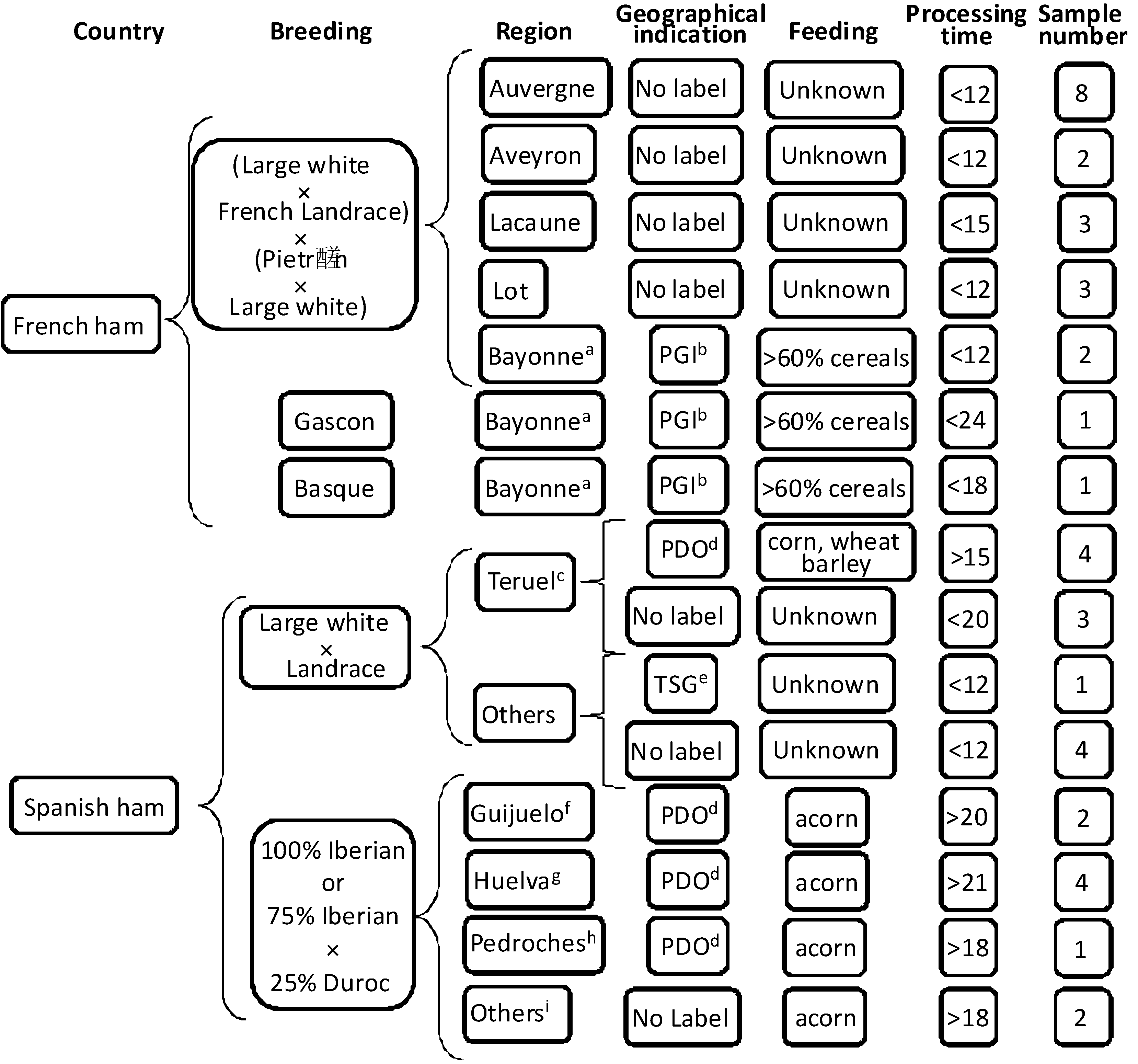
3.1. Samples
3.2. Analysis of Volatile Fraction by Gas-Chromatography
3.3. Response Factors
3.4. Odor Threshold of Volatile Compounds
3.5. GC-Olfactometry (GC-O)
3.6. Amino Acids and Related Compounds
3.7. Statistical Analyses
4. Conclusions
Acknowledgments
References
- Flores, J.; Toldrá, F. Curing: Processes and Applications. In Encyclopedia of Food Science, Food Technology and Nutrition; Macrae, R., Robinson, R., Sadler, M., Fullerlove, G., Eds.; Academic Press: London, UK, 1993; pp. 1277–1282. [Google Scholar]
- Flores, M.; Grimm, C.C.; Toldrá, F.; Spanier, A.M. Correlations of sensory and volatile compounds of Spanish “Serrano” dry-cured hams as a function of two processing times. J. Agric. Food Chem. 1997, 45, 2178–2186. [Google Scholar] [CrossRef]
- Toldrá, F. Proteolysis and lipolysis in flavour development of dry-cured meat products. Meat Sci. 1998, 49, 101–110. [Google Scholar] [CrossRef]
- Timón, M.L.; Ventanas, J.; Carrapiso, A.I.; Jurado, A.; García, C. Subcutaneous and intermuscular fat characterisation of dry-cured Iberian hams. Meat Sci. 2001, 58, 85–91. [Google Scholar] [CrossRef]
- Pastorelli, G.; Magni, S.; Rossi, R.; Pagliarini, E.; Baldini, P.; Dirinck, P.; Van Opstaele, F.; Corino, C. Influence of dietary fat, on fatty acid composition and sensory properties of dry-cured Parma ham. Meat Sci. 2003, 65, 571–580. [Google Scholar] [CrossRef]
- Berdagué, J.L.; Bonnaud, N.; Rousset, S.; Touraille, C. Influence of pig crossbreed on the composition, volatile compound content & flavour of dry cured ham. Meat Sci. 1993, 34, 119–129. [Google Scholar] [CrossRef]
- Ruiz, J.; Ventanas, R.; Cava, R.; Timón, M.L.; García, C. Sensory characteristics of Iberian ham: Influence of processing time and slice location. Food Res. Int. 1998, 31, 53–58. [Google Scholar] [CrossRef]
- García-González, D.L.; Tena, N.; Aparicio, R. Contributing to interpret sensory attributes qualifying Iberian hams from the volatile profile. Grasas Aceites 2009, 60, 277–283. [Google Scholar] [CrossRef]
- Gandemer, G. Dry cured ham quality as related to lipid quality of raw material and lipid changes during processing: A review. Grasas Aceites 2009, 60, 297–307. [Google Scholar] [CrossRef]
- Ruiz, J.; García, C.; Carmen Díaz, M.D.; Cava, R.; Florencio Tejeda, J.; Ventanas, J. Dry cured Iberian ham non-volatile components as affected by the length of the curing process. Food Res. Int. 1999, 32, 643–651. [Google Scholar] [CrossRef]
- Flores, M.; Aristoy, M.C.; Spanier, A.M.; Toldrá, F. Non-volatile components effects on quality of ‘serrano’ dry-cured ham as related to processing time. J. Food Sci. 1997, 62, 1235–1239. [Google Scholar] [CrossRef]
- Pérez-Juan, M.; Flores, M.; Toldrá, F. Generation of volatile flavour compounds as affected by the chemical composition of different dry-cured ham sections. Eur. Food Res. Technol. 2006, 222, 658–666. [Google Scholar] [CrossRef]
- Nguyen, T.H.D.; Wang, X.C. Volatile, taste components, and sensory characteristics of commercial brand oyster sauces: Comparisons and relationships. Int. J. Food Prop. 2012, 15, 518–535. [Google Scholar] [CrossRef]
- Toldrá, F. Dry-Cured Ham. In Handbook of Food Science Technology and Engineering; Hui, Y.H., Castell-Perez, E., Cunha, L.M., Guerrero-Legarreta, I., Liang, H.H., Lo, Y.M., Marshall, D.L., Nip, W.K., Shahidi, F., Sherkat, F., et al., Eds.; CRC Press: Boca Raton, FL, USA, 2006; Volume 4, pp. 164:1–164:11. [Google Scholar]
- Toldrá, F.; Aristoy, C.; Flores, M. Contribution of muscle aminopeptidases to flavor development in dry-cured ham. Food Res. Int. 2000, 33, 181–185. [Google Scholar] [CrossRef]
- Ramírez, R.; Cava, R. Volatile profiles of dry-cured meat products from three different iberian × duroc genotypes. J. Agric. Food Chem. 2007, 55, 1923–1931. [Google Scholar] [CrossRef]
- Sánchez-Peña, C.; Luna, G.; García-González, D.L.; Aparicio, R. Characterization of French and Spanish dry-cured hams: Influence of the volatiles from the muscles and the subcutaneous fat quantified by SPME-GC. Meat Sci. 2005, 69, 635–645. [Google Scholar] [CrossRef]
- Luna, G.; Aparicio, R.; García-González, D.L. A tentative characterization of white dry-cured hams from Teruel (Spain) by SPME-GC. Food Chem. 2006, 97, 621–630. [Google Scholar] [CrossRef]
- Sabio, E.; Vidal-Aragón, M.C.; Bernalte, M.J.; Gata, J.L. Volatile compounds present in six types of dry-cured ham from south European countries. Food Chem. 1998, 61, 493–503. [Google Scholar] [CrossRef]
- Buscailhon, S.; Berdagué, J.L.; Monin, G. Time-Related changes in volatile compounds of lean tissue during processing of French dry-cured ham. J. Sci. Food Agric. 1993, 63, 69–75. [Google Scholar] [CrossRef]
- Pham, A.J.; Schilling, M.W.; Mikel, W.B.; Williams, J.B.; Martin, J.M.; Coggins, P.C. Relationship between sensory descriptors, consumer acceptability and volatile flavor compound of American dry-cured ham. Meat Sci. 2008, 80, 728–737. [Google Scholar] [CrossRef]
- Gaspardo, B.; Procida, G.; Toso, B.; Stefano, B. Determination of volatile compounds in San Daniele ham using headspace GC-MS. Meat Sci. 2008, 80, 204–209. [Google Scholar]
- Armenteros, M.; Toldrá, F.; Aristoy, M.C.; Ventanas, J.; Estevez, M. Effect of the partial replacement of sodium chloride by other salts on the formation of volatile compounds during ripening of dry-cured ham. J. Agric. Food Chem. 2012, 60, 7607–7615. [Google Scholar] [CrossRef]
- Muriel, E.; Antequera, T.; Petrón, M.J.; Andrés, A.I.; Ruiz, J. Volatile compounds in Iberian dry-cured loin. Meat Sci. 2004, 68, 391–400. [Google Scholar] [CrossRef]
- Coutron-Gamboti, C.; Gandemer, G. Lipolysis and oxidation in subcutaneous adipose tissue during dry-cured ham processing. Food Chem. 1999, 64, 95–101. [Google Scholar] [CrossRef]
- Mottram, D.S. 1991. Meat. In Volatile Compounds in Food and Beverages; Maarse, H., Ed.; CRC: Belfast, UK, 1991; pp. 108–178. [Google Scholar]
- García, C.; Timón, M.L. Los compuestos responsables del “flavor” del jamón ibérico. Variaciones en los distintos tipos de jamones. In Tecnología del jamón Ibérico: de los sistemas tradicionales a la explotación racional del sabor y el aroma; Ventanas, J., Ed.; Ediciones Mundi-Prensa: Madrid, Spain, 2001; pp. 367–389. [Google Scholar]
- Wang, J.; Jin, G.; Zhang, W.; Ahn, D.I.; Zhang, J. Effect of curing salt content on lipid oxidation and volatile flavour compounds of dry-cured turkey ham. Food Sci. Technol. 2012, 48, 102–106. [Google Scholar]
- García, C.; Berdague, J.J.; Antequera, T.; Lopez-Bote, C.; Cordoba, J.J.; Ventanas, J. Volatile components of dry cured Iberian-ham. Food Chem. 1991, 41, 23–32. [Google Scholar] [CrossRef]
- Aparicio, R.; Morales, M.T.; Alonso, V. Relationship between volatile compounds and sensory attributes by statistical sensory wheel. J. Am. Oil Chem. Soc. 1996, 73, 1253–1264. [Google Scholar] [CrossRef]
- Aparicio, R.; Morales, M.T. Characterization of olive ripeness by green aroma compounds of virgin olive oil. J. Agric. Food Chem. 1998, 46, 1116–1122. [Google Scholar] [CrossRef]
- Morales, M.T.; Rios, J.J.; Aparicio, R. Changes in the volatile composition of virgin olive oil during oxidation: Flavor and off-flavors. J. Agric. Food Chem. 1997, 45, 2666–2673. [Google Scholar] [CrossRef]
- Grosch, W. Detection of potent odorants in foods by aroma extract dilution analysis. Trends Food Sci. Technol. 1993, 4, 68–73. [Google Scholar] [CrossRef]
- López, M.O.; de la Hoz, L.; Cambero, M.I.; Gallardo, E.; Reglero, G.; Ordoñez, J.A. Volatile compounds of dry hams from Iberian pig. Meat Sci. 1992, 31, 267–277. [Google Scholar] [CrossRef]
- Huan, Y.; Zhou, G.; Zhao, G.; Xu, X.; Peng, Z. Changes in flavor compound of dry-cured Chinese Jinhua ham during processing. Meat Sci. 2005, 71, 291–299. [Google Scholar] [CrossRef]
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Wnser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2003, 66, 21–32. [Google Scholar]
- Dirinck, P.; Opstaele, F.V.; Vandendriessche, F. Flavour differences between northern and southern European cured hams. Food Chem. 1997, 59, 511–521. [Google Scholar] [CrossRef]
- Gandemer, G. Lipids inmuscle and adipose tissues, changes during processing and sensory properties of meat products. Meat Sci. 2002, 62, 309–321. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Meat. In Food Chemistry, 4th ed.; Springer Verlag: Berlin, Germany, 2001; pp. 563–617. [Google Scholar]
- Lammers, M.; Dietze, K.; Terner, W. Headspace volatiles of dry-cured ham: A comparison of different manufacturing styles by SPME and GC/MS analysis. J. Food Proc. Preser. 2011, 35, 850–860. [Google Scholar] [CrossRef]
- Martín, A.; Córdoba, J.J.; Aranda, E.; Córdoba, M.G.; Asensio, M.A. Contribution of a selected fungal population to the volatile compounds on dry-cured ham. Int. J. Food Microbiol. 2006, 110, 8–18. [Google Scholar] [CrossRef]
- Berlitz, H.D.; Grosch, W. Food Chemistry; Springer-Verlag: Heidelberg, Germany, 1999. [Google Scholar]
- Brown, M.B.; Forsythe, A.B. Robust tests for the equality of variances. J. Am. Stat. Assoc. 1974, 69, 364–367. [Google Scholar] [CrossRef]
- European Communities (EC). Official Journal of the Commission of the European Communities, Regulation No. 97/C 22/03, 22 January 1997.
- Boletín de Aragón (BOA). Orden de 7 de mayo de 2012, del Consejero de Agricultura, Ganadería y Medio Ambiente, por la que se publica el pliego de condiciones modificado de la denominación de origen protegida “Jamón de Teruel” y se concede la protección nacional transitoria, 21 May 2012.
- European Communities (EC). Official Journal of the Commission of the European Communities, Regulation No. 2419/1999, 12 November 1999.
- Boletín Oficial del Estado (BOE). Regulation 29118/1993. ORDEN de 30 de noviembre de 1993 por la que se aprueba la modificación del Reglamento de la Denominación de Origen ‘Guijuelo’ y su Consejo Regulador, 7 December 1993.
- Boletín Oficial del Estado (BOE). Regulation 2139/1995. ORDEN de 12 de julio de 1995 por la que se aprueba el Reglamento de la Denominación de Origen ‘Jamón de Huelva’ y de su Consejo Regulador, 18 July 1995.
- Boletín Oficial de la Junta de Andalucía (BOJA). Orden de 6 de junio de 2012, por la que se aprueba el Reglamento del Consejo Regulador de la Denominación de Origen Protegida «Los Pedroches» y el pliego de condiciones de su producto, 15 June 2012.
- Boletín Oficial del Estado (BOE). Regulation 1469/2007. “Norma de calidad para la carne, el jamón, la paleta y la caña de lomo ibéricos, 3 November 2007.
- Mora, L.; Hernández-Cázares, A.S.; Sentandreu, M.A.; Toldrá, F. Creatine and creatinine evolution during the processing of dry-cured ham. Meat Sci. 2010, 84, 384–389. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
García-González, D.L.; Aparicio, R.; Aparicio-Ruiz, R. Volatile and Amino Acid Profiling of Dry Cured Hams from Different Swine Breeds and Processing Methods. Molecules 2013, 18, 3927-3947. https://doi.org/10.3390/molecules18043927
García-González DL, Aparicio R, Aparicio-Ruiz R. Volatile and Amino Acid Profiling of Dry Cured Hams from Different Swine Breeds and Processing Methods. Molecules. 2013; 18(4):3927-3947. https://doi.org/10.3390/molecules18043927
Chicago/Turabian StyleGarcía-González, Diego L., Ramón Aparicio, and Ramón Aparicio-Ruiz. 2013. "Volatile and Amino Acid Profiling of Dry Cured Hams from Different Swine Breeds and Processing Methods" Molecules 18, no. 4: 3927-3947. https://doi.org/10.3390/molecules18043927
APA StyleGarcía-González, D. L., Aparicio, R., & Aparicio-Ruiz, R. (2013). Volatile and Amino Acid Profiling of Dry Cured Hams from Different Swine Breeds and Processing Methods. Molecules, 18(4), 3927-3947. https://doi.org/10.3390/molecules18043927




