The Third Dimension of Reading the Sugar Code by Lectins: Design of Glycoclusters with Cyclic Scaffolds as Tools with the Aim to Define Correlations between Spatial Presentation and Activity
Abstract
:1. The Concept of the Sugar Code
2. Glycans as Bioactive Ligands for Lectins
| Type of fold | Example for lectin | Example for ligand |
|---|---|---|
| β-sandwich (jelly-roll) | (a) galectins | β-galactosides |
| (b) calnexin, calreticulin | Glc1Man9GlcNAc2 | |
| (c) ERGIC-53, VIP36, VIPL | ManxGlcNAc2 | |
| (d) CRD a of Fbs1 in SCF E3 ubiquitin ligase and peptide-N-glycanase | Man3GlcNAc2; mannopentaose | |
| (e) pentraxins | glycosaminoglycans, MOβDG, 3-sulfated Gal, GalNAc and GlcA, Man-6-phosphate | |
| (f) G-domains of the LNS family (laminin, agrin) | heparin | |
| C-type | asialoglycoprotein receptor, collectins, selectins | Fuc, Gal, GalNAc, Man, heparin tetrasaccharide |
| I-type (Ig fold) | N-CAM, TIM-3, siglecs | Man6GlcNAc2, HNK-1 epitope, α2,3/6-sialylated glycans |
| P-type | mannose-6-phosphate receptors (MR) and proteins with MR homology domain (erlectin, OS-9) | Man-6-phosphate, Man5,8GlcNAc2 |
| β-trefoil | (a) fibroblast growth factors | heparan sulfate |
| (b) cysteine-rich domain of C-type macrophage mannose receptor | GalNAc-4-sulfate in LacdiNAc | |
| (c) lectin domain in GalNAc-Tsb involved in mucin-type O-glycosylation | GalNAc | |
| (d) hemolytic lectin CEL-III of sea cucumber and lectin EW29 of earthworm | Gal | |
| β-propeller | (a) 4-bladed: tachylectin-3 | S-type lipopolysaccharide |
| (b) 5-bladed: tachylectin-2 | GlcNAc/GalNAc | |
| (c) 6-bladed: tachylectin-1 | KDO | |
| β-propeller | (a) 4-bladed: tachylectin-3 | S-type lipopolysaccharide |
| (b) 5-bladed: tachylectin-2 | GlcNAc/GalNAc | |
| (c) 6-bladed: tachylectin-1 | KDO | |
| β-prism I | secretory proteins zg16p/b | not defined |
| β-prism II | pufferfish (fugu) lectin | Man |
| β-barrel with jelly-roll topology | tachylectin-4, eel (Anguilla anguilla) agglutinin, X-epilectin | Fuc |
| fibrinogen-like domain | (a) ficolins | GlcNAc |
| (b) intelectins (mammalian, Xenopus) | Galf, pentoses | |
| (c) tachylectin-5 | N-acetylated sugars | |
| (d) slug (Limax flavus) lectin | sialic acid | |
| link module | CD44, TSG-6, LYVE-1, aggregating proteoglycans | hyaluronic acid |
| hevein-like domain | tachycytin and spider (Selenocosmia huwena) neurotoxin; cobra venom cardiotoxin | GalNAc; heparin-derived disaccharide |
| (β/α)8 barrel(glycoside hydrolase family 18) | YKL-40 (human cartilage glycoprotein-39; chitinase-like lectin) | (GlcNAc)n |
| short consensus repeat(complement control protein module) | factor H (complement regulator) | glycosaminoglycans, sialic acid |
3. Galectins: a Network of Bioeffectors
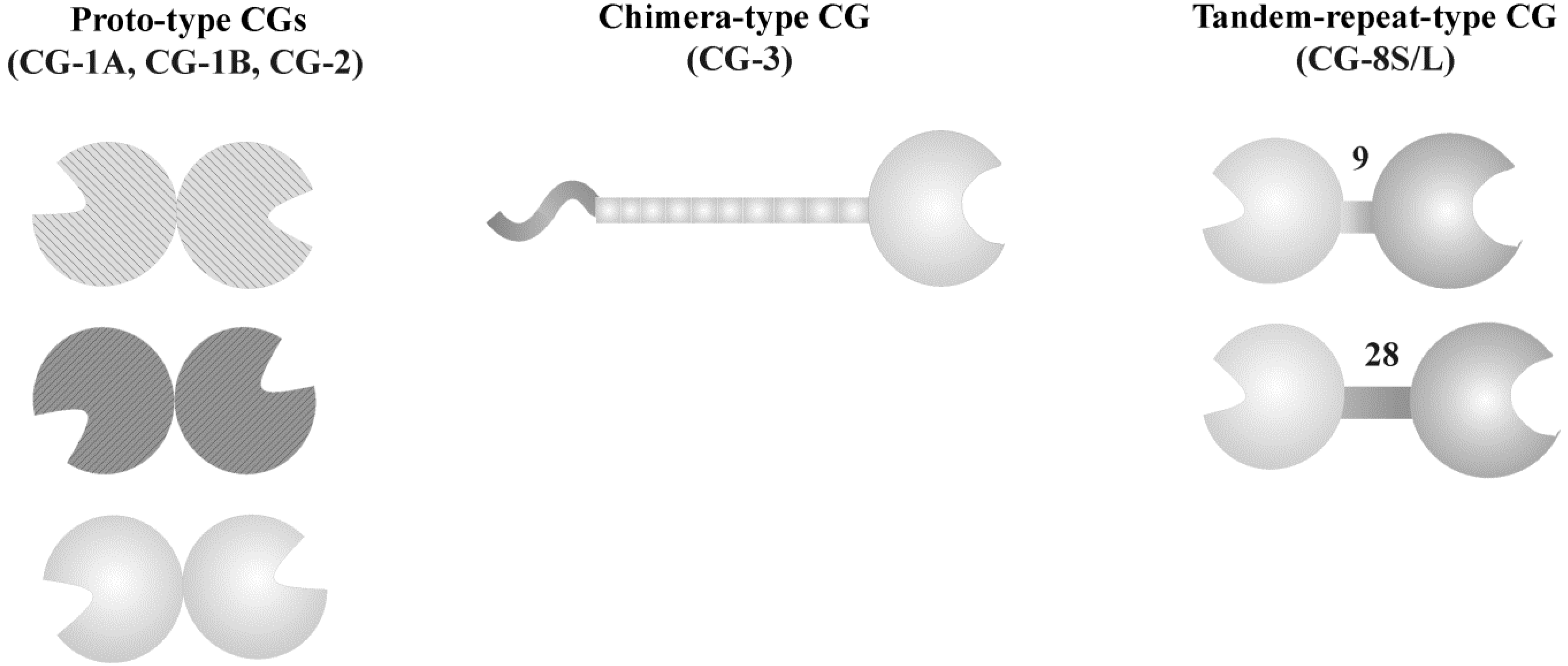
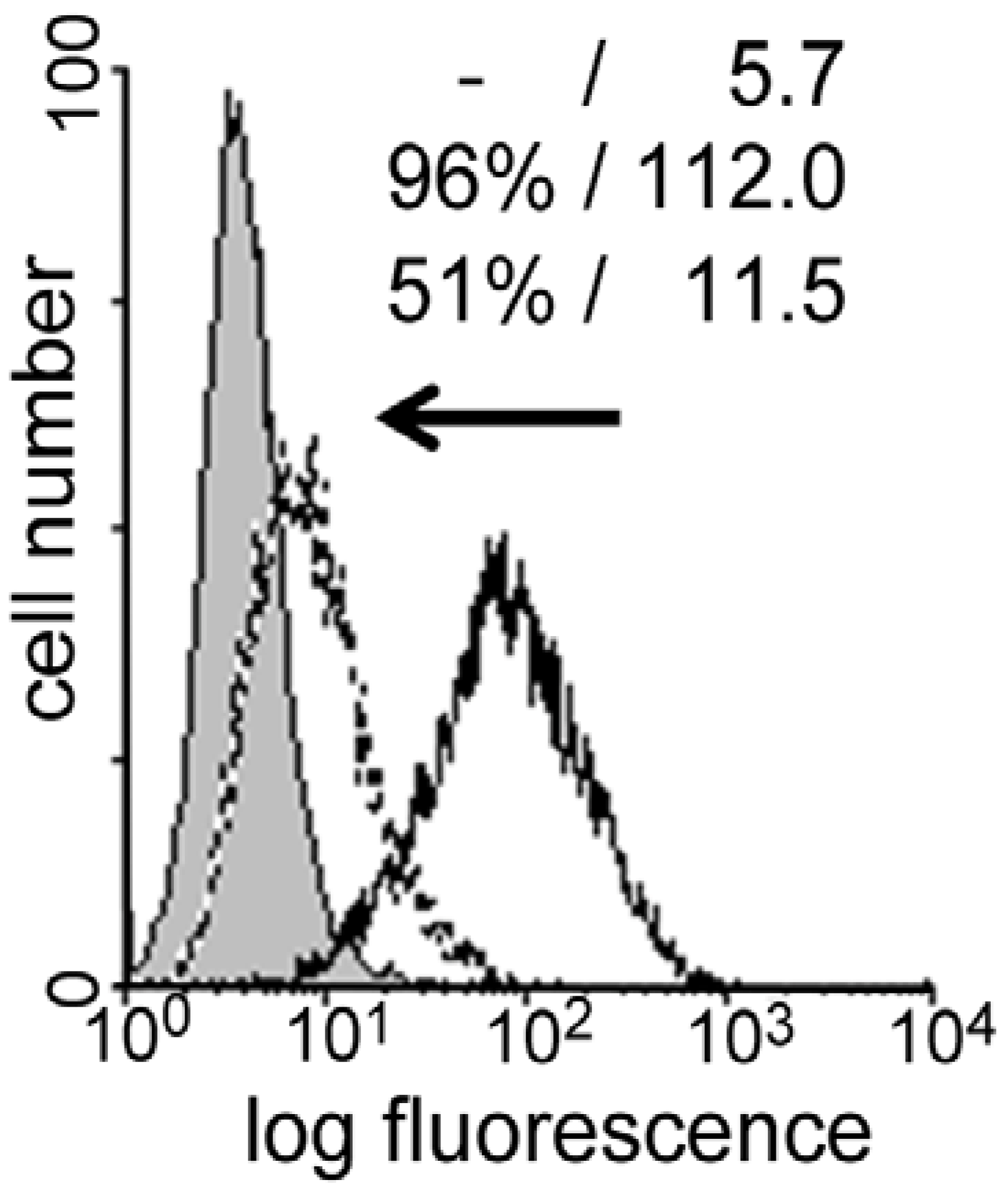
4. Cyclodextrins, Cyclic Decapeptides and Calixarenes
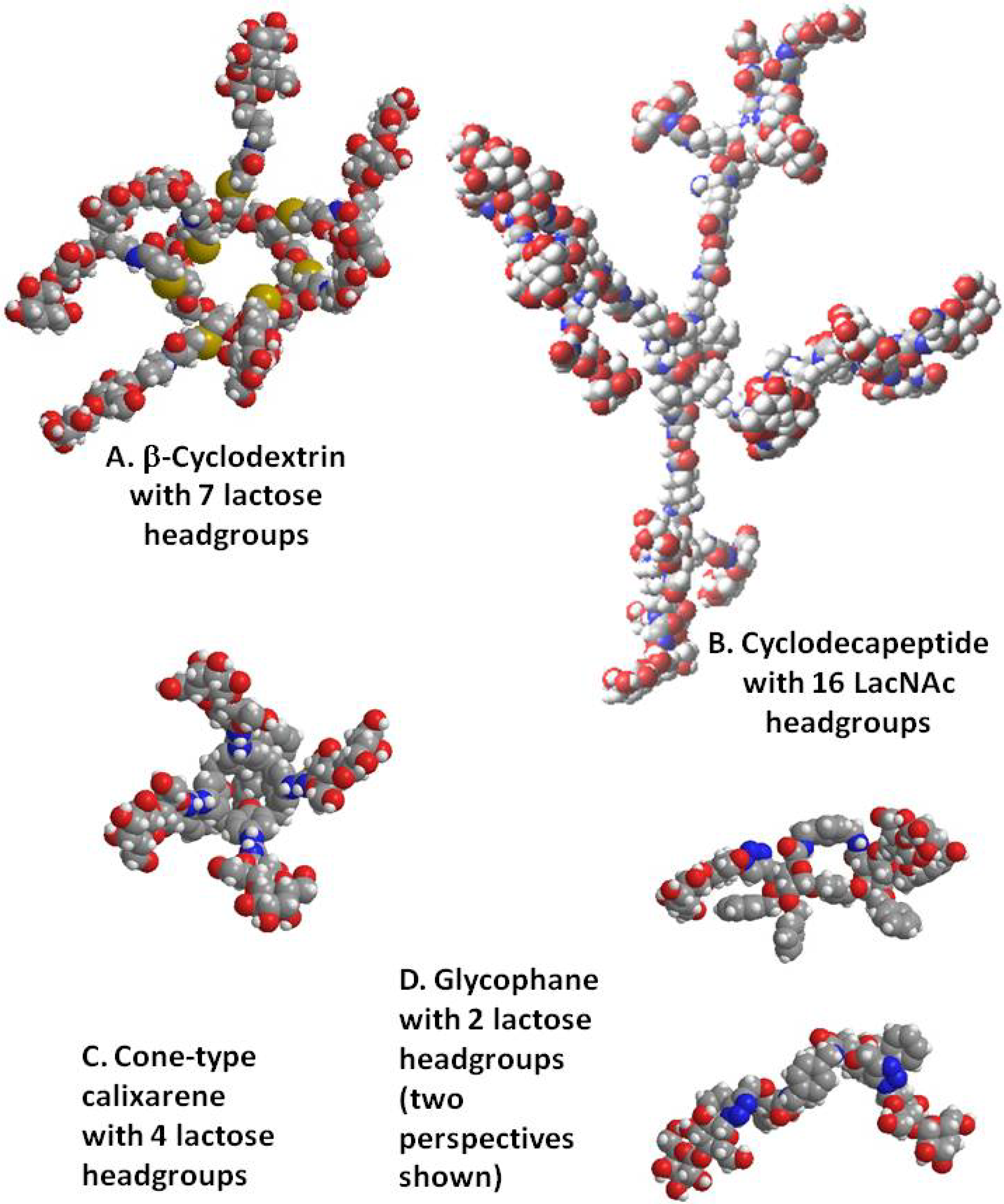
5. Glyco(cyclo)phanes
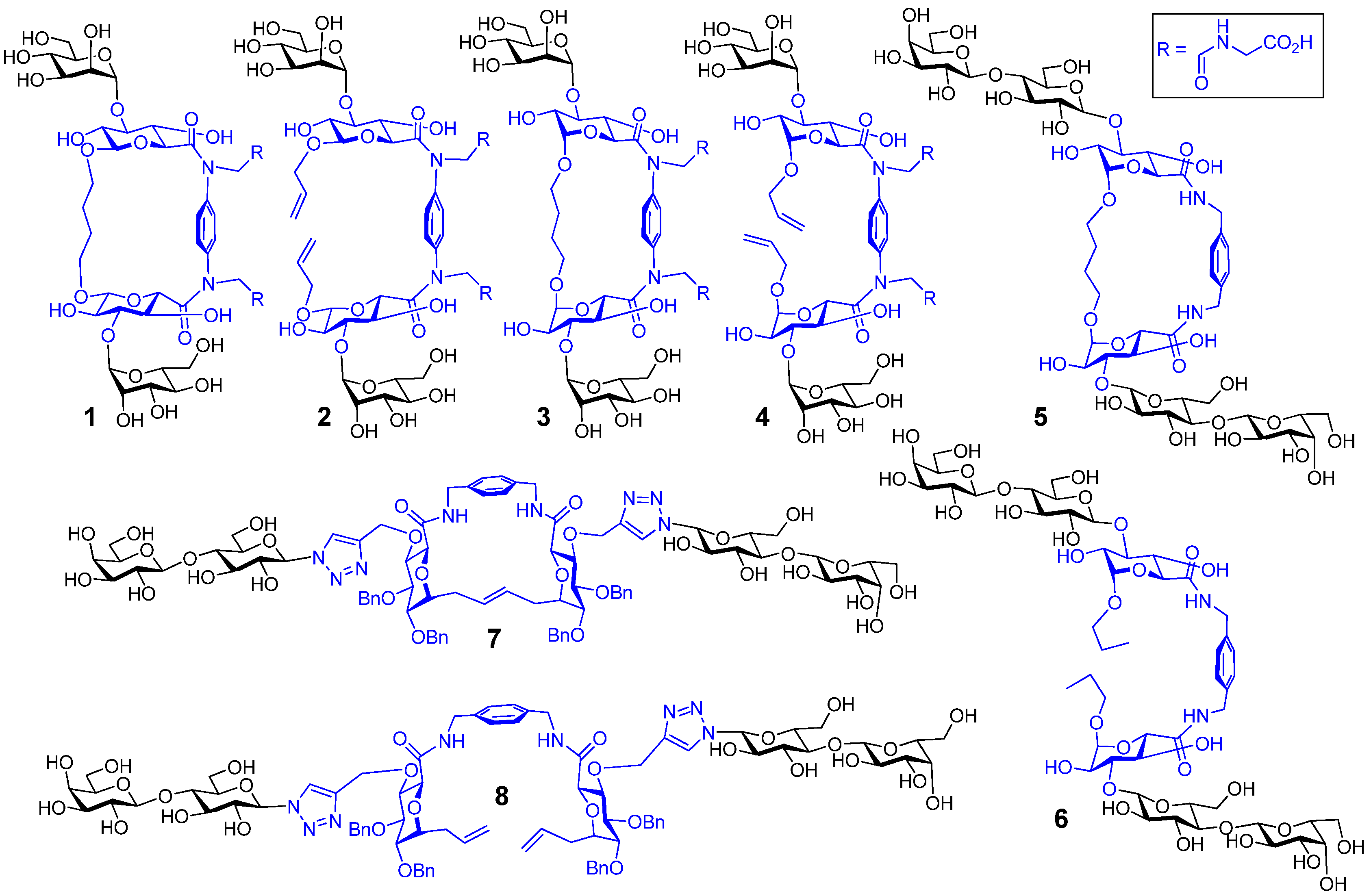
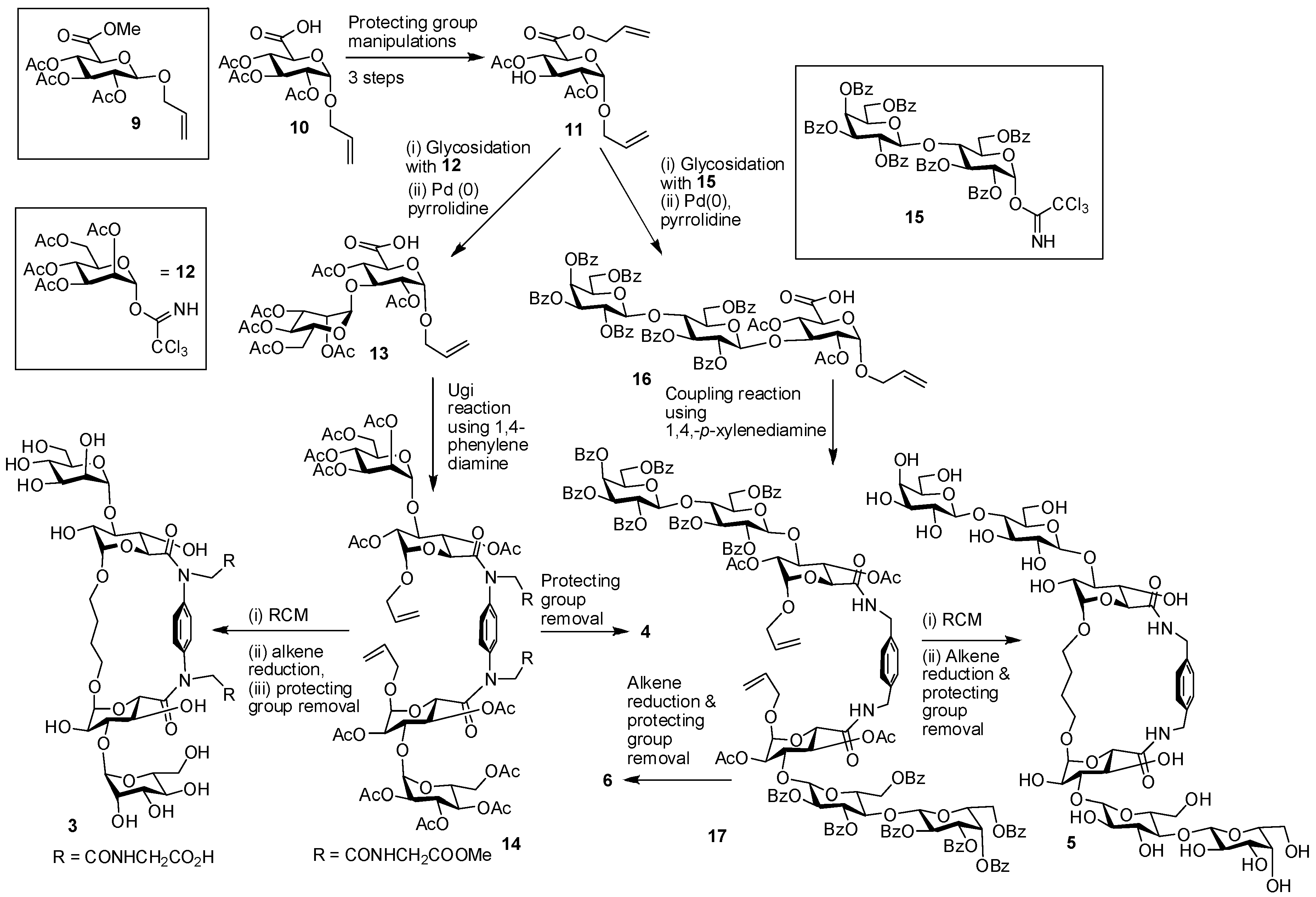
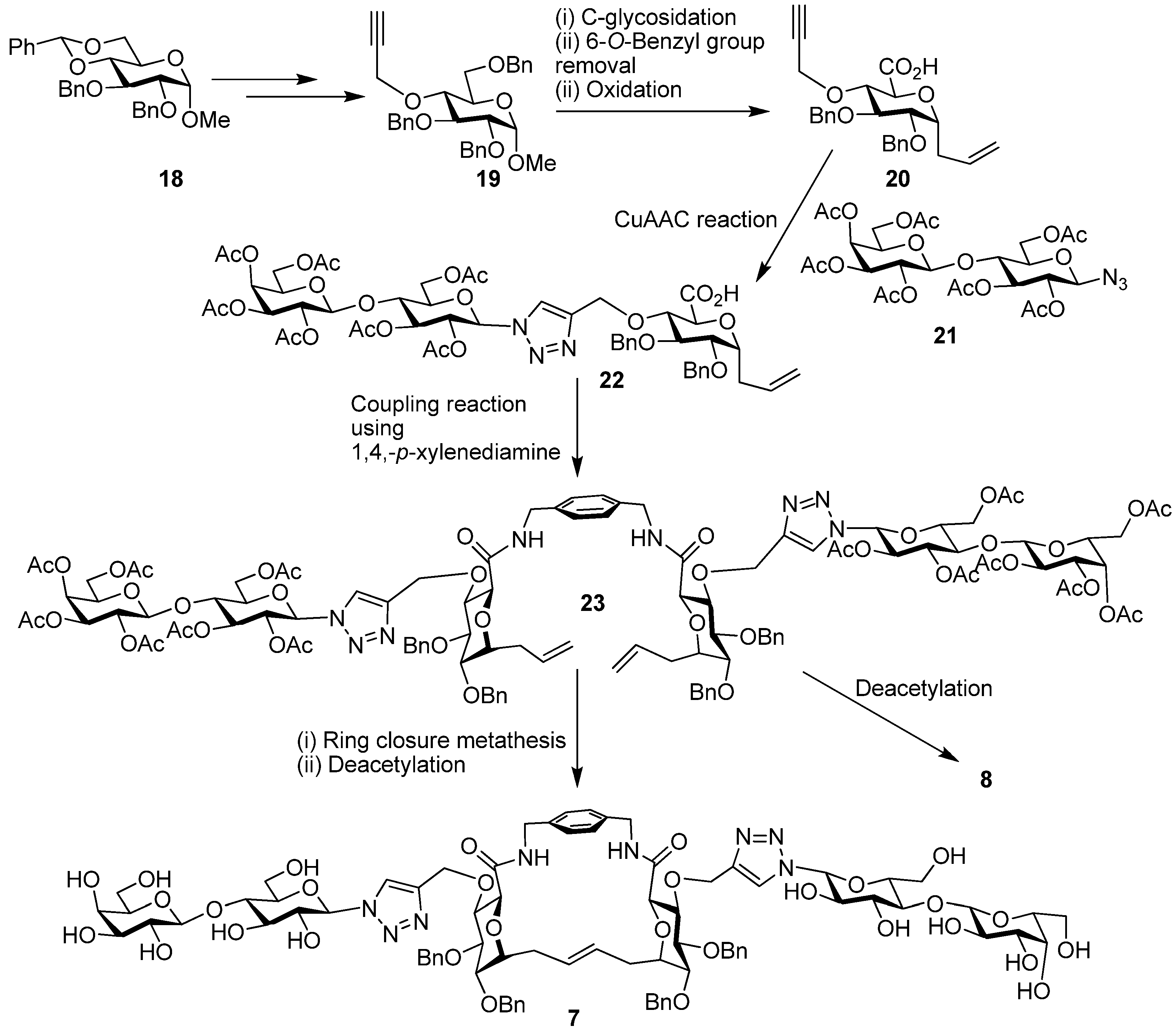


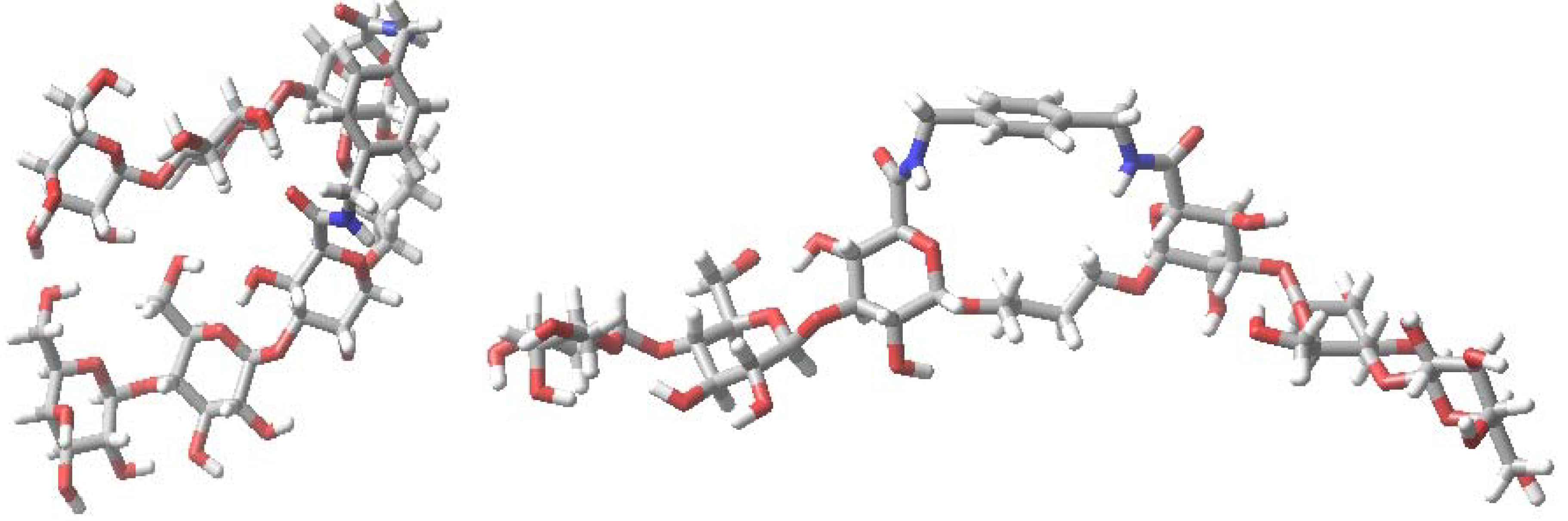
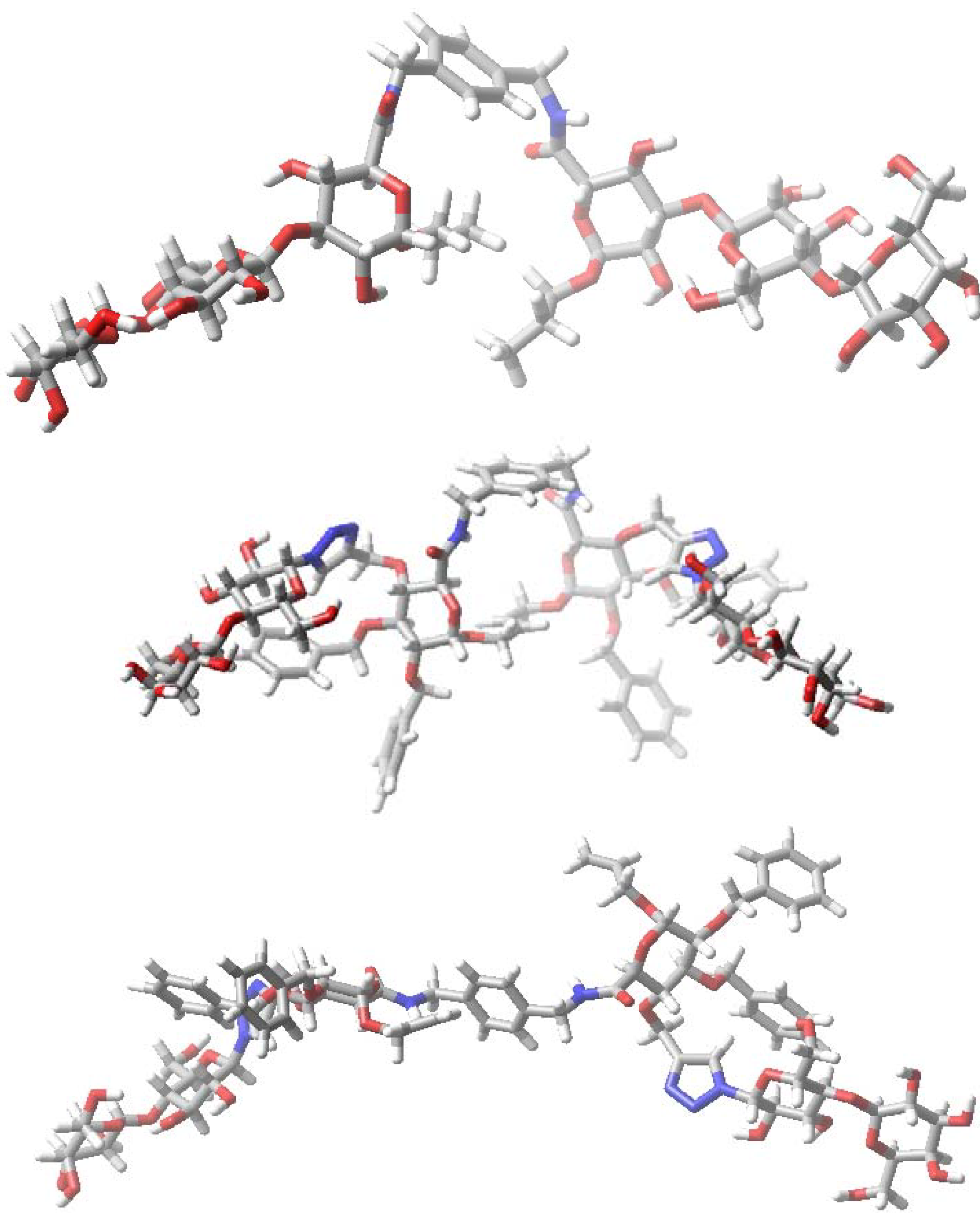
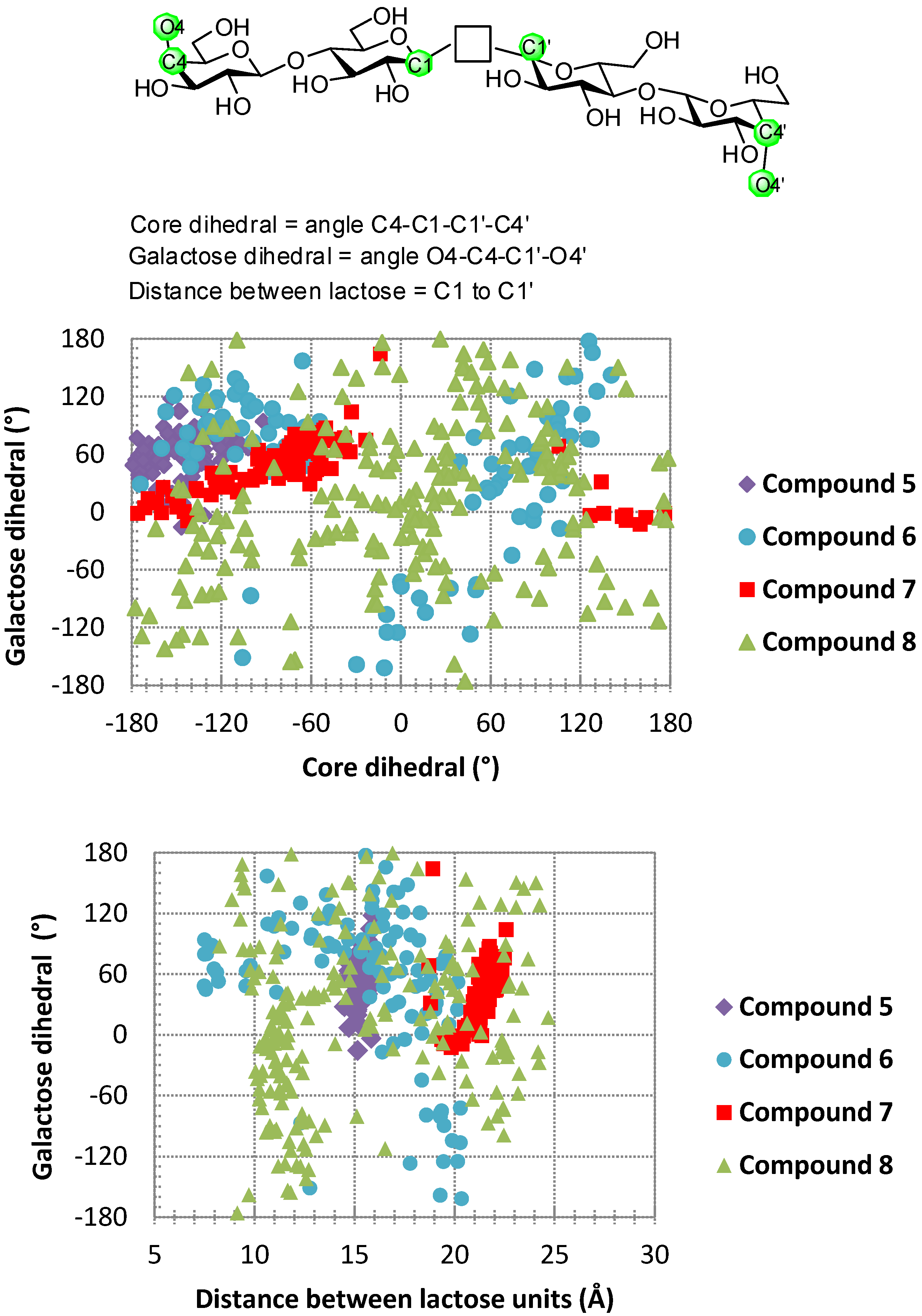
6. How to Optimize Inhibition, What to Expect
7. Conclusions
Acknowledgments
References
- Bond, A.E.; Row, P.E.; Dudley, E. Post-translation modification of proteins; Methodologies and applications in plant sciences. Phytochemistry 2011, 72, 975–996. [Google Scholar]
- Hunter, T. Why nature chose phosphate to modify proteins. Phil. Trans. R. Soc. B 2012, 367, 2513–2516. [Google Scholar] [CrossRef]
- Reuter, G.; Gabius, H.-J. Eukaryotic glycosylation: whim of nature or multipurpose tool? Cell. Mol. Life Sci. 1999, 55, 368–422. [Google Scholar] [CrossRef]
- Spiro, R.G. Protein glycosylation: Nature, Distribution, Enzymatic formation, And disease implications of glycopeptide bonds. Glycobiology 2002, 12, 43R–56R. [Google Scholar] [CrossRef]
- Zuber, C.; Roth, J. N-Glycosylation. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 87–110. [Google Scholar]
- Patsos, G.; Corfield, A. O-Glycosylation: Structural diversity and function. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 111–137. [Google Scholar]
- Wilson, I.B.H.; Paschinger, H.; Rendic, D. Glycosylation of model and “lower” organisms. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 139–154. [Google Scholar]
- Buddecke, E. Proteoglycans. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 199–216. [Google Scholar]
- Moremen, K.W.; Tiemeyer, M.; Nairn, A.V. Vertebrate protein glycosylation: Diversity, Synthesis and function. Nat. Rev. Mol. Cell Biol. 2012, 13, 448–462. [Google Scholar]
- Muthana, S.M.; Campbell, C.T.; Gildersleeve, J.C. Modifications of glycans: Biological significance and therapeutic opportunities. ACS Chem. Biol. 2012, 7, 31–43. [Google Scholar] [CrossRef]
- Vogt, G.; Chapgier, A.; Yang, K.; Chuzhanova, N.; Feinberg, J.; Fieschi, C.; Boisson-Dupuis, S.; Alcais, A.; Filipe-Santos, O.; Bustamante, J.; et al. Gains of glycosylation comprise an unexpectedly large group of pathogenic mutations. Nat. Genet. 2005, 37, 692–700. [Google Scholar]
- Vogt, G.; Vogt, B.; Chuzhanova, N.; Julenius, K.; Cooper, D.N.; Casanova, J.L. Gain-of-glycosylation mutations. Curr. Opin. Genet. Dev. 2007, 17, 245–251. [Google Scholar]
- Hennet, T. Diseases of glycosylation. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 365–383. [Google Scholar]
- Honke, K.; Taniguchi, N. Animal models to delineate glycan functionality. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 385–401. [Google Scholar]
- Nakagawa, H. Analytical aspects: Analysis of protein-bound glycans. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 71–83. [Google Scholar]
- Higgins, E. Carbohydrate analysis throughout the development of a protein therapeutic. Glycoconj. J. 2010, 27, 211–225. [Google Scholar] [CrossRef]
- Hansen, S.F.; Bettler, E.; Rinnan, A.; Engelsen, S.B.; Breton, C. Exploring genomes for glycosyltransferases. Mol. BioSyst. 2010, 6, 1773–1781. [Google Scholar] [CrossRef]
- Gabius, H.-J.; André, S.; Kaltner, H.; Siebert, H.-C. The sugar code: Functional lectinomics. Biochim. Biophys. Acta 2002, 1572, 165–177. [Google Scholar] [CrossRef]
- Cummings, R.D. The repertoire of glycan determinants in the human glycome. Mol. BioSyst. 2009, 5, 1087–1104. [Google Scholar] [CrossRef]
- Ma, B.; Simala-Grant, J.L.; Taylor, D.E. Fucosylation in prokaryotes and eukaryotes. Glycobiology 2006, 16, 158R–184R. [Google Scholar] [CrossRef]
- Aplin, J.D.; Jones, C.J. Fucose, Placental evolution and the glycocode. Glycobiology 2012, 22, 470–478. [Google Scholar] [CrossRef]
- Harduin-Lepers, A.; Mollicone, R.; Delannoy, P.; Oriol, R. The animal sialyltransferases and sialyltransferase-related genes: A phylogenetic approach. Glycobiology 2005, 15, 805–817. [Google Scholar] [CrossRef]
- Takashima, S. Characterization of mouse sialyltransferase genes: Their evolution and diversity. Biosci. Biotechnol. Biochem. 2008, 72, 1155–1167. [Google Scholar]
- Amano, M.; Eriksson, H.; Manning, J.C.; Detjen, K.M.; André, S.; Nishimura, S.-I.; Lehtiö, J.; Gabius, H.-J. Tumour suppressor p16(INK4a): Anoikis-favouring decrease in N/O-glycan/cell surface sialylation by down-regulation of enzymes in sialic acid biosynthesis in tandem in a pancreatic carcinoma model. FEBS J. 2012, 279, 4062–4080. [Google Scholar] [CrossRef]
- Liu, L.; Hirschberg, C.B. Developmental diseases caused by impaired nucleotide sugar transporters. Glycoconj. J. 2013, 30, 5–10. [Google Scholar] [CrossRef]
- Laine, R.A. The information-storing potential of the sugar code. In Glycosciences: Status and Perspectives; Gabius, H.-J., Gabius, S., Eds.; Chapman & Hall: London-Weinheim, UK/Germany, 1997; pp. 1–14. [Google Scholar]
- Gabius, H.-J. Biological information transfer beyond the genetic code: The sugar code. Naturwissenschaften 2000, 87, 108–121. [Google Scholar]
- Kopitz, J. Glycolipids. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 177–198. [Google Scholar]
- Unverzagt, C.; André, S.; Seifert, J.; Kojima, S.; Fink, C.; Srikrishna, G.; Freeze, H.; Kayser, K.; Gabius, H.-J. Structure-activity profiles of complex biantennary glycans with core fucosylation and with/without additional α2,3/α2,6-sialylation: Synthesis of neoglycoproteins and their properties in lectin assays, cell binding, and organ uptake. J. Med. Chem. 2002, 45, 478–491. [Google Scholar]
- André, S.; Unverzagt, C.; Kojima, S.; Frank, M.; Seifert, J.; Fink, C.; Kayser, K.; von der Lieth, C.-W.; Gabius, H.-J. Determination of modulation of ligand properties of synthetic complex-type biantennary N-glycans by introduction of bisecting GlcNAc in silico, in vitro and in vivo. Eur. J. Biochem. 2004, 271, 118–134. [Google Scholar]
- André, S.; Kozár, T.; Schuberth, R.; Unverzagt, C.; Kojima, S.; Gabius, H.-J. Substitutions in the N-glycan core as regulators of biorecognition: The case of core-fucose and bisecting GlcNAc moieties. Biochemistry 2007, 46, 6984–6995. [Google Scholar] [CrossRef]
- André, S.; Kozár, T.; Kojima, S.; Unverzagt, C.; Gabius, H.-J. From structural to functional glycomics: Core substitutions as molecular switches for shape and lectin affinity of N-glycans. Biol. Chem. 2009, 390, 557–565. [Google Scholar]
- Gabius, H.-J.; van de Wouwer, M.; André, S.; Villalobo, A. Down-regulation of the epidermal growth factor receptor by altering N-glycosylation: Emerging role of β1,4-galactosyltransferases. Anticancer Res. 2012, 32, 1565–1572. [Google Scholar]
- Quiocho, F.A. Carbohydrate-binding proteins: Tertiary structures and protein-sugar interactions. Annu. Rev. Biochem. 1986, 55, 287–315. [Google Scholar] [CrossRef]
- Lis, H.; Sharon, N. Lectins: Carbohydrate-specific proteins that mediate cellular recognition. Chem. Rev. 1998, 98, 637–674. [Google Scholar] [CrossRef]
- Gabius, H.-J.; André, S.; Jiménez-Barbero, J.; Romero, A.; Solís, D. From lectin structure to functional glycomics: Principles of the sugar code. Trends Biochem. Sci. 2011, 36, 298–313. [Google Scholar] [CrossRef]
- Gabius, H.-J. The how and why of Ca2+ involvement in lectin activity. Trends Glycosci. Glycotechnol. 2011, 23, 168–177. [Google Scholar] [CrossRef]
- Sperandio, M. Selectins and glycosyltransferases in leukocyte rolling in vivo. FEBS J. 2006, 273, 4377–4389. [Google Scholar] [CrossRef]
- André, S.; Sanchez-Ruderisch, H.; Nakagawa, H.; Buchholz, M.; Kopitz, J.; Forberich, P.; Kemmner, W.; Böck, C.; Deguchi, K.; Detjen, K.M.; et al. Tumor suppressor p16INK4a: Modulator of glycomic profile and galectin-1 expression to increase susceptibility to carbohydrate-dependent induction of anoikis in pancreatic carcinoma cells. FEBS J. 2007, 274, 3233–3256. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Z.H.; Gabius, H.-J.; Rohowsky-Kochan, C.; Ledeen, R.W.; Wu, G. Cross-linking of GM1 ganglioside by galectin-1 mediates regulatory T cell activity involving TRPC5 channel activation: Possible role in suppressing experimental autoimmune encephalomyelitis. J. Immunol. 2009, 182, 4036–4045. [Google Scholar] [CrossRef]
- Wu, G.; Lu, Z.H.; Gabius, H.-J.; Ledeen, R.W.; Bleich, D. Ganglioside GM1 deficiency in effector T cells from NOD mice induces resistance to regulatory T cell suppression. Diabetes 2011, 60, 2341–2349. [Google Scholar] [CrossRef]
- Kopitz, J.; Bergmann, M.; Gabius, H.-J. How adhesion/growth-regulatory galectins-1 and -3 attain cell specificity: Case study defining their target on neuroblastoma cells (SK-N-MC) and marked affinity regulation by affecting microdomain organization of the membrane. IUBMB Life 2010, 62, 624–628. [Google Scholar] [CrossRef]
- Fischer, C.; Sanchez-Ruderisch, H.; Welzel, M.; Wiedenmann, B.; Sakai, T.; André, S.; Gabius, H.-J.; Khachigian, L.; Detjen, K.; Rosewicz, S. Galectin-1 interacts with the α5β1 fibronectin receptor to restrict carcinoma cell growth via induction of p21 and p27. J. Biol. Chem. 2005, 280, 37266–37277. [Google Scholar] [CrossRef]
- Gabius, H.-J. Animal and human lectins. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 317–328. [Google Scholar]
- Hudgin, R.L.; Pricer, W.E., Jr.; Ashwell, G.; Stockert, R.J.; Morell, A.G. The isolation and properties of a rabbit liver binding protein specific for asialoglycoproteins. J. Biol. Chem. 1974, 249, 5536–5543. [Google Scholar]
- Lee, Y.C.; Townsend, R.R.; Hardy, M.R.; Lönngren, J.; Arnarp, J.; Haraldsson, M.; Lönn, H. Binding of synthetic oligosaccharides to the hepatic Gal/GalNAc lectin. J. Biol. Chem. 1983, 258, 199–202. [Google Scholar]
- Rogers, J.C.; Kornfeld, S. Hepatic uptake of proteins coupled to fetuin glycopeptide. Biochem. Biophys. Res. Commun. 1971, 45, 622–629. [Google Scholar] [CrossRef]
- Lee, R.T.; Lin, P.; Lee, Y.C. New synthetic cluster ligands for galactose/N-acetylgalactosamine-specific lectin of mammalian liver. Biochemistry 1984, 23, 4255–4261. [Google Scholar] [CrossRef]
- Lee, R.T.; Lee, Y.C. Preparation of cluster glycosides of N-acetylgalactosamine that have subnanomolar binding constants towards the mammalian hepatic Gal/GalNAc-specific receptor. Glycoconj. J. 1987, 4, 317–328. [Google Scholar] [CrossRef]
- André, S.; Kojima, S.; Gundel, G.; Russwurm, R.; Schratt, X.; Unverzagt, C.; Gabius, H.-J. Branching mode in complex-type triantennary N-glycans as regulatory element of their ligand properties. Biochim. Biophys. Acta 2006, 1760, 768–782. [Google Scholar] [CrossRef]
- Lee, R.T.; Lee, Y.C. Enhanced biochemical affinities of multivalent neoglycoconjugates. In Neoglycoconjugates. Preparation and Applications; Lee, Y.C., LeeR, T., Eds.; Academic Press: San Diego, CA, USA, 1994; pp. 23–50. [Google Scholar]
- Siebert, H.-C.; Adar, R.; Arango, R.; Burchert, M.; Kaltner, H.; Kayser, G.; Tajkhorshid, E.; von der Lieth, C.-W.; Kaptein, R.; Sharon, N.; et al. Involvement of laser photo CIDNP (chemically induced dynamic nuclear polarization)-reactive amino acid side chains in ligand binding by galactoside-specific lectins in solution. Eur. J. Biochem. 1997, 249, 27–38. [Google Scholar]
- Göhler, A.; Büchner, C.; André, S.; Doose, S.; Kaltner, H.; Gabius, H.-J. Sensing ligand binding to a clinically relevant lectin by tryptophan fluorescence anisotropy. Analyst 2011, 136, 5270–5276. [Google Scholar] [CrossRef] [Green Version]
- Göhler, A.; Büchner, C.; Doose, S.; Kaltner, H.; Gabius, H.-J. Analysis of homodimeric avian and human galectins by two methods based on fluorescence spectroscopy: Different structural alterations upon oxidation and ligand binding. Biochimie 2012, 94, 2649–2655. [Google Scholar] [CrossRef]
- López-Lucendo, M.F.; Solís, D.; André, S.; Hirabayashi, J.; Kasai, K.-I.; Kaltner, H.; Gabius, H.-J.; Romero, A. Growth-regulatory human galectin-1: Crystallographic characterisation of the structural changes induced by single-site mutations and their impact on the thermodynamics of ligand binding. J. Mol. Biol. 2004, 343, 957–970. [Google Scholar] [CrossRef]
- Kasai, K.-I.; Hirabayashi, J. Galectins: A family of animal lectins that decipher glycocodes. J. Biochem. 1996, 119, 1–8. [Google Scholar] [CrossRef]
- Cooper, D.N.W. Galectinomics: Finding themes in complexity. Biochim. Biophys. Acta 2002, 1572, 209–231. [Google Scholar] [CrossRef]
- Kaltner, H.; Gabius, H.-J. A toolbox of lectins for translating the sugar code: the galectin network in phylogenesis and tumors. Histol. Histopathol. 2012, 27, 397–416. [Google Scholar]
- Gupta, D.; Kaltner, H.; Dong, X.; Gabius, H.-J.; Brewer, C.F. Comparative cross-linking activities of lactose-specific plant and animal lectins and a natural lactose-binding immunoglobulin G fraction from human serum with asialofetuin. Glycobiology 1996, 6, 843–849. [Google Scholar] [CrossRef]
- Kopitz, J.; von Reitzenstein, C.; André, S.; Kaltner, H.; Uhl, J.; Ehemann, V.; Cantz, M.; Gabius, H.-J. Negative regulation of neuroblastoma cell growth by carbohydrate-dependent surface binding of galectin-1 and functional divergence from galectin-3. J. Biol. Chem. 2001, 276, 35917–35923. [Google Scholar] [CrossRef]
- Sanchez-Ruderisch, H.; Fischer, C.; Detjen, K.M.; Welzel, M.; Wimmel, A.; Manning, J.C.; André, S.; Gabius, H.-J. Tumor suppressor p16INK4a: Downregulation of galectin-3, an endogenous competitor of the pro-anoikis effector galectin-1, in a pancreatic carcinoma model. FEBS J. 2010, 277, 3552–3563. [Google Scholar] [CrossRef]
- Gabius, H.-J.; Brehler, R.; Schauer, A.; Cramer, F. Localization of endogenous lectins in normal human breast, benign breast lesions and mammary carcinomas. Virch. Arch. [Cell. Pathol.] 1986, 52, 107–115. [Google Scholar] [CrossRef]
- Kayser, K.; Höft, D.; Hufnagl, P.; Caselitz, J.; Zick, Y.; André, S.; Kaltner, H.; Gabius, H.-J. Combined analysis of tumor growth pattern and expression of endogenous lectins as a prognostic tool in primary testicular cancer and its lung metastases. Histol. Histopathol. 2003, 18, 771–779. [Google Scholar]
- Saussez, S.; de Leval, L.; Decaestecker, C.; Sirtaine, N.; Cludts, S.; Duray, A.; Chevalier, D.; André, S.; Gabius, H.-J.; Remmelink, M.; Leroy, X. Galectin fingerprinting in Warthin’s tumors: Lectin-based approach to trace its origin? Histol. Histopathol. 2010, 25, 541–550. [Google Scholar]
- Remmelink, M.; de Leval, L.; Decaestecker, C.; Duray, A.; Crompot, E.; Sirtaine, N.; Andre, S.; Kaltner, H.; Leroy, X.; Gabius, H.J.; et al. Quantitative immunohistochemical fingerprinting of adhesion/growth-regulatory galectins in salivary gland tumours: Divergent profiles with diagnostic potential. Histopathology 2011, 58, 543–556. [Google Scholar] [CrossRef]
- André, S.; Jarikote, D.V.; Yan, D.; Vincenz, L.; Wang, G.N.; Kaltner, H.; Murphy, P.V.; Gabius, H.-J. Synthesis of bivalent lactosides and their activity as sensors for differences between lectins in inter- and intrafamily comparisons. Bioorg. Med. Chem. Lett. 2012, 22, 313–318. [Google Scholar]
- Den, H.; Malinzak, D.A. Isolation and properties of β-d-galactoside-specific lectin from chick embryo thigh muscle. J. Biol. Chem. 1977, 252, 5444–5448. [Google Scholar]
- Beyer, E.C.; Zweig, S.E.; Barondes, S.H. Two lactose-binding lectins from chicken tissues. Purified lectin from intestine is different from those in liver and muscle. J. Biol. Chem. 1980, 255, 4236–4239. [Google Scholar]
- Oda, Y.; Kasai, K.-I. Purification and characterization of β-galactoside-binding lectin from chick embryonic skin. Biochim. Biophys. Acta 1983, 761, 237–245. [Google Scholar] [CrossRef]
- Kaltner, H.; Solís, D.; Kopitz, J.; Lensch, M.; Lohr, M.; Manning, J.C.; Mürnseer, M.; Schnölzer, M.; André, S.; Sáiz, J.L.; et al. Proto-type chicken galectins revisited: Characterization of a third protein with distinctive hydrodynamic behaviour and expression pattern in organs of adult animals. Biochem. J. 2008, 409, 591–599. [Google Scholar]
- Kaltner, H.; Solís, D.; André, S.; Lensch, M.; Manning, J.C.; Mürnseer, M.; Saíz, J.L.; Gabius, H.-J. Unique chicken tandem-repeat-type galectin: Implications of alternative splicing and a distinct expression profile compared to those of the three proto-type proteins. Biochemistry 2009, 48, 4403–4416. [Google Scholar] [CrossRef]
- Kaltner, H.; Kübler, D.; López-Merino, L.; Lohr, M.; Manning, J.C.; Lensch, M.; Seidler, J.; Lehmann, W.D.; André, S.; Solís, D.; et al. Toward comprehensive analysis of the galectin network in chicken: Unique diversity of galectin-3 and comparison of its localization profile in organs of adult animals to the other four members of this lectin family. Anat. Rec. 2011, 294, 427–444. [Google Scholar] [CrossRef]
- Ledeen, R.W.; Wu, G.; André, S.; Bleich, D.; Huet, G.; Kaltner, H.; Kopitz, J.; Gabius, H.-J. Beyond glycoproteins as galectin counterreceptors: Tumor/effector T cell growth control via ganglioside GM1. Ann. NY Acad. Sci. 2012, 1253, 206–221. [Google Scholar] [CrossRef]
- Fulton, D.A.; Stoddart, J.F. Neoglycoconjugates based on cyclodextrins and calixarenes. Bioconjug. Chem. 2001, 12, 655–672. [Google Scholar] [CrossRef]
- Houseman, B.T.; Mrksich, M. Model systems for studying polyvalent carbohydrate binding interactions. Top. Curr. Chem. 2002, 218, 1–44. [Google Scholar] [CrossRef]
- Mellet, C.O.; Defaye, J.; Fernandez, J.M.G. Multivalent cyclooligosaccharides: Versatile carbohydrate clusters with dual role as molecular receptors and lectin ligands. Chem. Eur. J. 2002, 8, 1982–1990. [Google Scholar] [CrossRef]
- Furuike, T.; Aiba, S.; Nishimura, S.I. A highly practical synthesis of cyclodextrin-based glycoclusters having enhanced affinity with lectins. Tetrahedron 2000, 56, 9909–9915. [Google Scholar] [CrossRef]
- André, S.; Kaltner, H.; Furuike, T.; Nishimura, S.-I.; Gabius, H.-J. Persubstituted cyclodextrin-based glycoclusters as inhibitors of protein-carbohydrate recognition using purified plant and mammalian lectins and wild-type and lectin-gene-transfected tumor cells as targets. Bioconjug. Chem. 2004, 15, 87–98. [Google Scholar]
- Ahmad, N.; Gabius, H.-J.; André, S.; Kaltner, H.; Sabesan, S.; Roy, R.; Liu, B.; Macaluso, F.; Brewer, C.F. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J. Biol. Chem. 2004, 279, 10841–10847. [Google Scholar] [CrossRef]
- André, S.; Liu, B.; Gabius, H.-J.; Roy, R. First demonstration of differential inhibition of lectin binding by synthetic tri-and tetravalent glycoclusters from cross-coupling of rigidified 2-propynyl lactoside. Org. Biomol. Chem. 2003, 1, 3909–3916. [Google Scholar] [CrossRef]
- André, S.; Specker, D.; Bovin, N.V.; Lensch, M.; Kaltner, H.; Gabius, H.-J.; Wittmann, V. Carbamate-linked lactose: Design of clusters and evidence for selectivity to block binding of human lectins to (neo)glycoproteins with increasing degree of branching and to tumor cells. Bioconjug.e Chem. 2009, 20, 1716–1728. [Google Scholar]
- Nelson, A.; Belitsky, J.M.; Vidal, S.; Joiner, C.S.; Baum, L.G.; Stoddart, J.F. A self-assembled multivalent pseudopolyrotaxane for binding galectin-1. J. Am. Chem. Soc. 2004, 126, 11914–11922. [Google Scholar] [CrossRef]
- Belitsky, J.M.; Nelson, A.; Hernandez, J.D.; Baum, L.G.; Stoddart, J.F. Multivalent interactions between lectins and supramolecular complexes: Galectin-1 and self-assembled pseudopolyrotaxanes. Chem. Biol. 2007, 14, 1140–1151. [Google Scholar]
- Wittmann, V.; Seeberger, S. Combinatorial solid-phase synthesis of multivalent cyclic neoglycopeptides. Angew. Chem. Int. Ed. 2000, 39, 4348–4352. [Google Scholar]
- Renaudet, O. Recent advances on cyclopeptide-based glycoclusters. Mini-Rev. Org. Chem. 2008, 5, 274–286. [Google Scholar]
- André, S.; Renaudet, O.; Bossu, I.; Dumy, P.; Gabius, H.-J. Cyclic neoglycodecapeptides: how to increase their inhibitory activity and selectivity on lectin/toxin binding to a glycoprotein and cells. J. Pept. Sci. 2011, 17, 427–437. [Google Scholar] [CrossRef]
- Dam, T.K.; Gabius, H.-J.; André, S.; Kaltner, H.; Lensch, M.; Brewer, C.F. Galectins bind to the multivalent glycoprotein asialofetuin with enhanced affinities and a gradient of decreasing binding constants. Biochemistry 2005, 44, 12564–12571. [Google Scholar] [CrossRef]
- Kopitz, J.; Ballikaya, S.; André, S.; Gabius, H.-J. Ganglioside GM1/galectin-dependent growth regulation in human neuroblastoma cells: Special properties of bivalent galectin-4 and significance of linker length for ligand selection. Neurochem. Res. 2012, 37, 1267–1276. [Google Scholar] [CrossRef]
- André, S.; Sansone, F.; Kaltner, H.; Casnati, A.; Kopitz, J.; Gabius, H.-J.; Ungaro, R. Calix[n]arene-based glycoclusters: Bioactivity of thiourea-linked galactose/lactose moieties as inhibitors of binding of medically relevant lectins to a glycoprotein and cell-surface glycoconjugates and selectivity among human adhesion/growth-regulatory galectins. ChemBioChem 2008, 9, 1649–1661. [Google Scholar] [CrossRef]
- Cecioni, S.; Matthews, S.E.; Blanchard, H.; Praly, J.P.; Imberty, A.; Vidal, S. Synthesis of lactosylated glycoclusters and inhibition studies with plant and human lectins. Carbohydr. Res. 2012, 356, 132–141. [Google Scholar] [CrossRef]
- André, S.; Grandjean, C.; Gautier, F.M.; Bernardi, S.; Sansone, F.; Gabius, H.-J.; Ungaro, R. Combining carbohydrate substitutions at bioinspired positions with multivalent presentation towards optimising lectin inhibitors: Case study with calixarenes. Chem. Commun. (Camb) 2011, 47, 6126–6128. [Google Scholar] [CrossRef]
- Bukownik, R.R.; Wilcox, C.S. Synthetic receptors. 3,6-Anhydro-7-benzenesulfonamido-1,7-dideoxy-4,5-O-isopropylidene-D-altro-hept-1-ynitol: A useful component for the preparation of chiral water-soluble cyclophanes based on carbohydrate precursors. J. Org. Chem. 1988, 53, 463–467. [Google Scholar] [CrossRef]
- Jiménez-Barbero, J.; Junquera, E.; Martin-Pastor, M.; Sharma, S.; Vicent, C.; Penades, S. Molecular recognition of carbohydrates using a synthetic receptor. A model system to understand the stereoselectivity of a carbohydrate-carbohydrate interaction in water. J. Am. Chem. Soc. 1995, 117, 11198–11204. [Google Scholar] [CrossRef]
- Gulder, T.; Baran, P.S. Strained cyclophane natural products: Macrocyclization at its limits. Nat. Prod. Rep. 2012, 29, 899–934. [Google Scholar] [CrossRef]
- Velasco-Torrijos, T.; Murphy, P.V. Synthesis and conformational analysis of novel water soluble macrocycles incorporating carbohydrates, including a β-cyclodextrin mimic. Tetrahedron- Asymmetr. 2005, 16, 261–272. [Google Scholar] [CrossRef]
- Murphy, P.V.; Müller-Bunz, H.; Velasco-Torrijos, T. The crystal structure of a cyclic glycolipid reveals a carbohydrate-carbohydrate interaction interface. Carbohydr. Res. 2005, 340, 1437–1440. [Google Scholar] [CrossRef]
- Murphy, P.V. Peptidomimetics, glycomimetics and scaffolds from carbohydrate building blocks. Eur. J. Org. Chem. 2007, 4177–4187. [Google Scholar] [CrossRef]
- Polakova, M.; Pitt, N.; Tosin, M.; Murphy, P.V. Glycosidation reactions of silyl ethers with conformationally inverted donors derived from glucuronic acid: Stereoselective synthesis of glycosides and 2-deoxyglycosides. Angew. Chem. Int. Ed. 2004, 43, 2518–2521. [Google Scholar] [CrossRef]
- Bradley, H.; Fitzpatrick, G.; Glass, W.K.; Kunz, H.; Murphy, P.V. Application of Ugi reactions in synthesis of divalent neoglycoconjugates: Evidence that the sugars are presented in restricted conformation. Org. Lett. 2001, 3, 2629–2632. [Google Scholar] [CrossRef]
- Ugi, I.; Werner, B.; Dömling, A. The chemistry of isocyanides, their multicomponent reactions and their librarie. Molecules 2003, 8, 53–66. [Google Scholar] [CrossRef]
- Leyden, R.; Velasco-Torrijos, T.; André, S.; Gouin, S.; Gabius, H.-J.; Murphy, P.V. Synthesis of bivalent lactosides based on terephthalamide, N,N'-diglucosylterephthalamide, and glycophane scaffolds and assessment of their inhibitory capacity on medically relevant lectins. J. Org. Chem. 2009, 74, 9010–9026. [Google Scholar] [CrossRef]
- Tornoe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(i)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Trnka, T.M.; Grubbs, R.H. The development of L2X2Ru=CHR olefin metathesis catalysts: An organometallic success story. Acc. Chem. Res. 2001, 34, 18–29. [Google Scholar] [CrossRef]
- Jarikote, D.V.; Murphy, P.V. Metathesis and macrocycles with embedded carbohydrates. Eur. J. Org. Chem. 2010, 4959–4970. [Google Scholar] [CrossRef]
- Mohamadi, F.; Richards, N.G.J.; Guida, W.C.; Liskamp, R.; Lipton, M.; Caufield, C.; Chang, G.; Hendrickson, T.; Still, W.C. Macromodel: An integrated software system for modeling organic and bioorganic molecules using molecular mechanics. J. Comput. Chem. 1990, 11, 440–467. [Google Scholar] [CrossRef]
- André, S.; Velasco-Torrijos, T.; Leyden, R.; Gouin, S.; Tosin, M.; Murphy, P.V.; Gabius, H.-J. Phenylenediamine-based bivalent glycocyclophanes: Synthesis and analysis of the influence of scaffold rigidity and ligand spacing on lectin binding in cell systems with different glycomic profiles. Org. Biomol. Chem. 2009, 7, 4715–4725. [Google Scholar] [CrossRef]
- Wang, G.-N.; André, S.; Gabius, H.-J.; Murphy, P.V. Bi- to tetravalent glycoclusters: Synthesis, structure-activity profiles as lectin inhibitors and impact of combining both valency and headgroup tailoring on selectivity. Org. Biomol. Chem. 2012, 10, 6893–6907. [Google Scholar] [CrossRef]
- Ahmed, H.; Allen, H.J.; Sharma, A.; Matta, K.L. Human splenic galaptin: Carbohydrate-binding specificity and characterization of the combining site. Biochemistry 1990, 29, 5315–5319. [Google Scholar] [CrossRef]
- Lee, R.T.; Ichikawa, Y.; Allen, H.J.; Lee, Y.C. Binding characteristics of galactoside-binding lectin (galaptin) from human spleen. J. Biol. Chem. 1990, 265, 7864–7871. [Google Scholar]
- André, S.; Giguère, D.; Dam, T.K.; Brewer, C.F.; Gabius, H.-J.; Roy, R. Synthesis and screening of a small glycomimetic library for inhibitory activity on medically relevant galactoside-specific lectins in assays of increasing biorelevance. New J. Chem. 2010, 34, 2229–2240. [Google Scholar] [CrossRef]
- Giguère, D.; André, S.; Bonin, M.A.; Bellefleur, M.A.; Provencal, A.; Cloutier, P.; Pucci, B.; Roy, R.; Gabius, H.-J. Inhibitory potential of chemical substitutions at bioinspired sites of β-D-galactopyranose on neoglycoprotein/cell surface binding of two classes of medically relevant lectins. Bioorg. Med. Chem. 2011, 19, 3280–3287. [Google Scholar] [CrossRef]
- Sparrow, C.; Leffler, H.; Barondes, S.H. Multiple soluble α-galactoside-binding lectins from human lung. J. Biol. Chem. 1987, 262, 7383–7390. [Google Scholar]
- Solís, D.; Romero, A.; Kaltner, H.; Gabius, H.-J.; Díaz-Mauriño, T. Different architecture of the combining sites of two chicken galectins revealed by chemical-mapping studies with synthetic ligand derivatives. J. Biol. Chem. 1996, 271, 12744–12748. [Google Scholar]
- Ahmad, N.; Gabius, H.-J.; Kaltner, H.; André, S.; Kuwabara, I.; Liu, F.-T.; Oscarson, S.; Norberg, T.; Brewer, C.F. Thermodynamic binding studies of cell surface carbohydrate epitopes to galectins-1, -3 and -7. Evidence for differential binding specificities. Can. J. Chem. 2002, 80, 1096–1104. [Google Scholar] [CrossRef]
- Hirabayashi, J.; Hashidate, T.; Arata, Y.; Nishi, N.; Nakamura, T.; Hirashima, M.; Urashima, T.; Oka, T.; Futai, M.; Müller, W.E.G.; et al. Oligosaccharide specificity of galectins: A search by frontal affinity chromatography. Biochim. Biophys. Acta 2002, 1572, 232–254. [Google Scholar] [CrossRef]
- Stowell, S.R.; Arthur, C.M.; Slanina, K.A.; Horton, J.R.; Smith, D.F.; Cummings, R.D. Dimeric Galectin-8 induces phosphatidylserine exposure in leukocytes through polylactosamine recognition by the C-terminal domain. J. Biol. Chem. 2008, 283, 20547–20559. [Google Scholar] [CrossRef]
- Solís, D.; Maté, M.J.; Lohr, M.; Ribeiro, J.P.; López-Merino, L.; André, S.; Buzamet, E.; Cañada, F.J.; Kaltner, H.; Lensch, M.; et al. N-Domain of human adhesion/growth-regulatory galectin-9: Preference for distinct conformers and non-sialylated N-glycans and detection of ligand-induced structural changes in crystal and solution. Int. J. Biochem. Cell Biol. 2010, 42, 1019–1029. [Google Scholar] [CrossRef]
- Krzeminski, M.; Singh, T.; André, S.; Lensch, M.; Wu, A.M.; Bonvin, A.M.J.J.; Gabius, H.-J. Human galectin-3 (Mac-2 antigen): Defining molecular switches of affinity to natural glycoproteins, structural and dynamic aspects of glycan binding by flexible ligand docking and putative regulatory sequences in the proximal promoter region. Biochim. Biophys. Acta 2011, 1810, 150–161. [Google Scholar] [CrossRef]
- Martín-Santamaría, S.; André, S.; Buzamet, E.; Caraballo, R.; Fernández-Cureses, G.; Morando, M.; Ribeiro, J.P.; Ramírez-Gualito, K.; de Pascual-Teresa, B.; Cañada, F.J.; et al. Strategic combination of binding studies and NMR spectroscopy and detection of selectivity between a plant toxin and human lectins. Org. Biomol. Chem. 2011, 9, 5445–5455. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.C.; Ribeiro, J.P.; Roldós, V.; Martín-Santamaría, S.; Cañada, F.J.; Nesmelova, I.V.; André, S.; Pang, M.; Klyosov, A.A.; Baum, L.G.; et al. Structural aspects of binding of α-linked digalactosides to human galectin-1. Glycobiology 2011, 21, 1627–1641. [Google Scholar] [CrossRef]
- Vokhmyanina, O.A.; Rapoport, E.M.; André, S.; Severov, V.V.; Ryzhov, I.; Pazynina, G.V.; Korchagina, E.; Gabius, H.-J.; Bovin, N.V. Comparative study of the glycan specificities of cell-bound human tandem-repeat-type galectins-4, -8 and -9. Glycobiology 2012, 22, 1207–1217. [Google Scholar] [CrossRef]
- Delacour, D.; Gouyer, V.; Zanetta, J.-P.; Drobecq, H.; Leteurtre, E.; Grard, G.; Moreau-Hannedouche, O.; Maes, E.; Pons, A.; André, S.; et al. Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells. J. Cell Biol. 2005, 169, 491–501. [Google Scholar] [CrossRef]
- Stechly, L.; Morelle, W.; Dessein, A.F.; André, S.; Grard, G.; Trinel, D.; Dejonghe, M.J.; Leteurtre, E.; Drobecq, H.; Trugnan, G.; et al. Galectin-4-regulated delivery of glycoproteins to the brush border membrane of enterocyte-like cells. Traffic 2009, 10, 438–450. [Google Scholar] [CrossRef]
- Wu, A.M.; Wu, J.H.; Liu, J.-H.; Singh, T.; André, S.; Kaltner, H.; Gabius, H.-J. Effects of polyvalency of glycotopes and natural modifications of human blood group ABH/Lewis sugars at the Galβ1-terminated core saccharides on the binding of domain-I of recombinant tandem-repeat-type galectin-4 from rat gastrointestinal tract (G4-N). Biochimie 2004, 86, 317–326. [Google Scholar] [CrossRef]
- Oscarson, S. The chemist’s way to synthesize glycosides. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 31–51. [Google Scholar]
- Chabre, Y.M.; Roy, R. The chemist’s way to prepare multivalency. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 53–70. [Google Scholar]
- André, S.; Cejas Ortega, P.J.; Alamino Perez, M.; Roy, R.; Gabius, H.-J. Lactose-containing starburst dendrimers: Influence of dendrimer generation and binding-site orientation of receptors (plant/animal lectins and immunoglobulins) on binding properties. Glycobiology 1999, 9, 1253–1261. [Google Scholar] [CrossRef]
- Griegel, S.; Rajewsky, M.F.; Ciesiolka, T.; Gabius, H.J. Endogenous sugar receptor (lectin) profiles of human retinoblastoma and retinoblast cell lines analyzed by cytological markers, affinity chromatography and neoglycoprotein-targeted photolysis. Anticancer Res. 1989, 9, 723–730. [Google Scholar]
- Bartoloni, M.; Dominguez, B.E.; Dragoni, E.; Richichi, B.; Fragai, M.; André, S.; Gabius, H.-J.; Arda, A.; Luchinat, C.; Jiménez-Barbero, J.; et al. Targeting matrix metalloproteinases: Design of a bifunctional inhibitor for presentation by tumour-associated galectins. Chem. Eur. J. 2013, 19, 1896–1902. [Google Scholar] [CrossRef]
- Habermann, F.A.; André, S.; Kaltner, H.; Kübler, D.; Sinowatz, F.; Gabius, H.-J. Galectins as tools for glycan mapping in histology: Comparison of their binding profiles to the bovine zona pellucida by confocal laser scanning microscopy. Histochem. Cell Biol. 2011, 135, 539–552. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; André, S.; Janko, C.; Kaltner, H.; Kopitz, J.; Gabius, H.-J.; Herrmann, M. Adhesion/growth-regulatory galectins in the human eye: Localization profiles and tissue reactivities as a standard to detect disease-associated alterations. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 1169–1180. [Google Scholar] [CrossRef]
- Smetana, K., Jr.; André, S.; Kaltner, H.; Kopitz, J.; Gabius, H.-J. Context-dependent multifunctionality of galectin-1: A challenge for defining the lectin as therapeutic target. Exp. Opin. Ther. Targ. 2013, 17, 379–392. [Google Scholar] [CrossRef]
- Rorive, S.; Belot, N.; Decaestecker, C.; Lefranc, F.; Gordower, L.; Micik, S.; Maurage, C.-A.; Kaltner, H.; Ruchoux, M.-M.; Danguy, A.; et al. Galectin-1 is highly expressed in human gliomas with relevance for modulation of invasion of tumor astrocytes into the brain parenchyma. Glia 2001, 33, 241–255. [Google Scholar] [CrossRef]
- Roda, O.; Ortiz-Zapater, E.; Martínez-Bosch, N.; Gutiérrez-Gallego, R.; Vila-Perelló, M.; Ampurdanés, C.; Gabius, H.-J.; André, S.; Andreu, D.; Real, F.X.; et al. Galectin-1 is a novel functional receptor for tissue plasminogen activator in pancreatic cancer. Gastroenterology 2009, 136, 1379–1390. [Google Scholar] [CrossRef]
- Gabius, H.-J.; Bodanowitz, S.; Schauer, A. Endogenous sugar-binding proteins in human breast tissue and benign and malignant breast lesions. Cancer 1988, 61, 1125–1131. [Google Scholar] [CrossRef]
- Gabius, H.-J.; Gabius, S.; Zemlyanukhina, T.V.; Bovin, N.V.; Brinck, U.; Danguy, A.; Joshi, S.S.; Kayser, K.; Schottelius, J.; Sinowatz, F.; et al. Reverse lectin histochemistry: Design and application of glycoligands for detection of cell and tissue lectins. Histol. Histopathol. 1993, 8, 369–383. [Google Scholar]
- Kayser, K.; Bovin, N.V.; Korchagina, E.Y.; Zeilinger, C.; Zeng, F.-Y.; Gabius, H.-J. Correlation of expression of binding sites for synthetic blood group A-, B-, and H-trisaccharides and for sarcolectin with survival of patients with bronchial carcinom. Eur. J. Cancer 1994, 30A, 653–657. [Google Scholar]
- Gready, J.N.; Zelensky, A.N. Routes in lectin evolution: case study on the C-type lectin-like domains. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 329–346. [Google Scholar]
- André, S.; Kopitz, J.; Kaltner, H.; Villalobo, A.; Gabius, H.-J. Glycans as functional markers in malignancy? In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 419–432. [Google Scholar]
- Schwartz-Albiez, R. Inflammation and glycosciences. In The Sugar Code. Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 447–467. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Murphy, P.V.; André, S.; Gabius, H.-J. The Third Dimension of Reading the Sugar Code by Lectins: Design of Glycoclusters with Cyclic Scaffolds as Tools with the Aim to Define Correlations between Spatial Presentation and Activity. Molecules 2013, 18, 4026-4053. https://doi.org/10.3390/molecules18044026
Murphy PV, André S, Gabius H-J. The Third Dimension of Reading the Sugar Code by Lectins: Design of Glycoclusters with Cyclic Scaffolds as Tools with the Aim to Define Correlations between Spatial Presentation and Activity. Molecules. 2013; 18(4):4026-4053. https://doi.org/10.3390/molecules18044026
Chicago/Turabian StyleMurphy, Paul V., Sabine André, and Hans-Joachim Gabius. 2013. "The Third Dimension of Reading the Sugar Code by Lectins: Design of Glycoclusters with Cyclic Scaffolds as Tools with the Aim to Define Correlations between Spatial Presentation and Activity" Molecules 18, no. 4: 4026-4053. https://doi.org/10.3390/molecules18044026
APA StyleMurphy, P. V., André, S., & Gabius, H.-J. (2013). The Third Dimension of Reading the Sugar Code by Lectins: Design of Glycoclusters with Cyclic Scaffolds as Tools with the Aim to Define Correlations between Spatial Presentation and Activity. Molecules, 18(4), 4026-4053. https://doi.org/10.3390/molecules18044026





