Effect of Erica sp. Honey against Microorganisms of Clinical Importance: Study of the Factors Underlying this Biological Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Pollen Analysis
2.2. Phenolics and Flavonoids
2.3. Quantification of the Hydrogen Peroxide
2.4. Antimicrobial Activity
 ), artificial honey (■); against four bacteria (Bacillus cereus sp., Escherichia coli sp., Pseudomonas aeruginosa sp. and Staphylococcus aureus sp.) and four yeasts (Candida famata sp., Cryptococcus neoformans sp., Candida krusei sp. and Candida albicans sp.).
), artificial honey (■); against four bacteria (Bacillus cereus sp., Escherichia coli sp., Pseudomonas aeruginosa sp. and Staphylococcus aureus sp.) and four yeasts (Candida famata sp., Cryptococcus neoformans sp., Candida krusei sp. and Candida albicans sp.).
 ), artificial honey (■); against four bacteria (Bacillus cereus sp., Escherichia coli sp., Pseudomonas aeruginosa sp. and Staphylococcus aureus sp.) and four yeasts (Candida famata sp., Cryptococcus neoformans sp., Candida krusei sp. and Candida albicans sp.).
), artificial honey (■); against four bacteria (Bacillus cereus sp., Escherichia coli sp., Pseudomonas aeruginosa sp. and Staphylococcus aureus sp.) and four yeasts (Candida famata sp., Cryptococcus neoformans sp., Candida krusei sp. and Candida albicans sp.).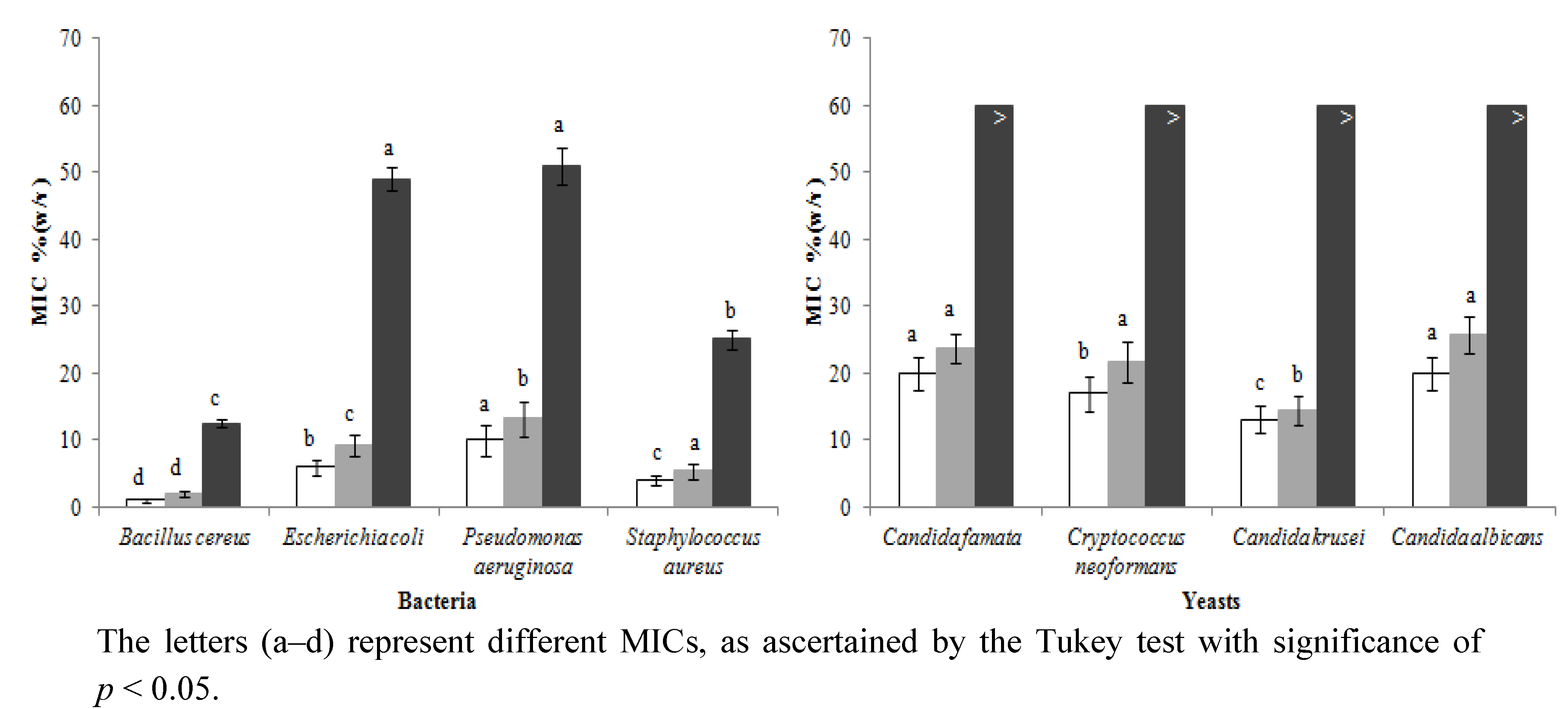
| Variable | MIC % (w/v) | |
|---|---|---|
| Microorganisms | B. cereus | 1.51 ± 0.90 d |
| S. aureus | 4.73 ± 2.09 c | |
| E. coli | 7.60 ± 3.69 b | |
| P. aeruginosa | 11.63 ± 5.23 a | |
| Catalase | Absence | 5.25 ± 4.29 b |
| Presence | 7.48 ± 5.47 a | |
| Effects significance | Microorganisms | *** |
| Catalase | *** | |
| Interaction | NS | |
| Variable | MIC % (w/v) | |
|---|---|---|
| Microorganisms | C. krusei | 14.33 ± 5.68 c |
| C. neoformans | 19.33 ± 8.15 b | |
| C. famata | 22.38 ± 9.14 a | |
| C. albicans | 23.33 ± 10.31 a | |
| Catalase | Absence | 17.52 ± 7.30 b |
| Presence | 22.17 ± 10.32 a | |
| Effects Significance | Microorganisms | *** |
| Catalase | *** | |
| Interaction | NS | |
| Correlation | B. cereus | S. aureus | E. coli | P. aeruginosa |
|---|---|---|---|---|
| MIC versus phenols | −0.115 | −0.098 | 0.043 | 0.111 |
| MIC versus flavonoids | 0.365 *** | −0.146 | 0.089 | 0.010 |
| MIC versus sugars | −0.066 | 0.177 | −0.103 | −0.030 |
| Correlation | C. krusei | C. neoformans | C. famata | C. albicans |
|---|---|---|---|---|
| MIC versus phenols | −0.109 | −0.117 | 0.011 | −0.080 |
| MIC versus flavonoids | 0.026 | −0.087 | 0.309 ** | 0.1356 |
| MIC versus sugars | −0.130 | −0.113 | −0.068 | −0.067 |
3. Experimental
3.1. Chemicals and Apparatus
3.2. Honey Samples
3.3. Sample Floral-Type Identification
3.4. Bioactive Compounds Quantification
3.4.1. Total Phenolic Content (TPC)
3.4.2. Total Flavonoid Content (TFC)
3.5. Hydrogen Peroxide Assay
3.6. Antimicrobial Activity Tests
3.6.1. Microbial Species
3.6.2. Culture Media and Inoculums
3.6.3. Antimicrobial Assay
3.6.4. Artificial Honey
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Tenover, F.C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Infect. Control 2006, 34, 3–10. [Google Scholar] [CrossRef]
- Livermore, D.M. Bacterial resistance: Origins, Epidemiology, and impact. Clin. Infect. Dis. 2003, 36, 11–23. [Google Scholar] [CrossRef]
- Cutler, J.E.; Deepe, G.S., Jr.; Klein, B.S. Advances in combating fungal diseases: vaccines on the threshold. Nat. Rev. Micro. 2007, 5, 13–28. [Google Scholar] [CrossRef]
- Brudzynski, K.; Lannigan, R. Mechanism of honey bacteriostatic action against MRSA and VRE involves hydroxyl radicals generated from honey’s hydrogen peroxide. Front. Microbiol. 2012, 3, 1–8. [Google Scholar]
- Brudzynski, C.; Abubaker, A.; St-Martin, L.; Castle, A. Re-examining the role of hydrogen peroxide in bacteriostatic and bactericidal activities of honey. Front. Microbiol. 2011, 2, 213. [Google Scholar]
- Sajid, M.; Azim, M.K. Characterization of the nematicidal activity of natural honey. J. Agric. Food Chem. 2012, 60, 7428–7434. [Google Scholar] [CrossRef]
- Molan, P. Why honey is effective as a medicine. Bee World 2001, 82, 22–40. [Google Scholar]
- Estevinho, L.M.; Afonso, S.E.; Feás, X. Antifungal effect of lavender honey against Candida albicans, Candida krusei and Cryptococcus neoformans. J. Food Sci. Technol. 2011, 48, 640–643. [Google Scholar] [CrossRef]
- Irish, J.; Carter, D.A.; Shokohi, T.; Blair, S. Honey has an antifungal effect against Candida species. Med. Mycol. 2006, 44, 289–291. [Google Scholar]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Honey: A Novel Antioxidant. Molecules 2012, 17, 4400–4423. [Google Scholar] [CrossRef]
- Ghashm, A.A.; Othman, N.H.; Khattak, M.N.; Ismail, N.M.; Saini, R. Antiproliferative effect of Tualang honey on oral squamous cell carcinoma and osteosarcoma cell lines. BMC Complement. Altern. Med. 2010, 14, 49. [Google Scholar]
- Haynes, J.S. Achieving clinical outcomes: The use of honey. Wound Essentials 2011, 6, 14–19. [Google Scholar]
- Molan, P.C. Potential of honey in the treatment of wounds and burns. Am. J. Clin. Dermatol. 2001, 2, 13–19. [Google Scholar] [CrossRef]
- Lusby, P.E.; Coombes, A.L.; Wilkinson, J.M. Bactericidal activity of different honeys against pathogenic bacteria. Arch. Med. Res. 2005, 36, 464–467. [Google Scholar] [CrossRef]
- Kwakman, P.H.; te Velde, A.A.; de Boer, L.; Speijer, D.; Vandenbroucke-Grauls, C.M.; Zaat, S.A. How honey kills bacteria. FASEB J. 2010, 24, 2576–2582. [Google Scholar] [CrossRef]
- Chen, C.; Campbell, L.T.; Blair, S.E.; Carter, D.A. The effect of standard heat and filtration processing procedures on antimicrobial activity and hydrogen peroxide levels in honey. Front. Microbiol. 2012, 3, 1–7. [Google Scholar]
- Tucak, Z.; Periskić, M.; Beslo, D.; Tucak, I. Influence of the beehive type on the quality of honey. Coll. Antropol. 2004, 28, 463–467. [Google Scholar]
- Witczak, M.; Juszczak, L.; Gałkowska, D. Non-Newtonian behaviour of heather honey. J. Food Eng. 2011, 104, 532–537. [Google Scholar] [CrossRef]
- Harfani, H.; Bouanani, N.; Aziz, M.; Caid, H.; Ghalim, N.; Amrani, S. The hypolipidaemic activity of aqueous Erica multiflora flowers extract in Triton WR-1339 induced hyperlipidaemic rats: a comparison with fenofibrate. J. Ethnopharmacol. 2007, 109, 156–160. [Google Scholar] [CrossRef]
- Akkol, E.; Yesxilada, E.; Güvenc, A. Valuation of anti-inflammatory and antinociceptive activities of Erica species native to Turkey. J. Ethnopharmacol. 2007, 116, 251–257. [Google Scholar]
- Tuzlaci, E.; Aymaz, E.P. Turkish folk medicinal plants, Part IV: Gönen (Balikesir). Fitoterapia 2001, 72, 323–343. [Google Scholar] [CrossRef]
- Andrade, P.B.; Amaral, M.T.; Isabel, P.; Carvalho, J.; Seabra, R.; Cunha, A. Pysicochemical attributes and pollen spectrum of Portuguese heather honeys. Food Chem. 1999, 66, 503–510. [Google Scholar] [CrossRef]
- Pires, J.; Estevinho, M.L.; Feás, X.; Cantalapiedra, J.; Iglesias, A. Pollen spectrum and physico-chemical attributes of heather (Erica sp.) honeys of north Portugal. J. Sci. Food Agric. 2009, 89, 1862–1870. [Google Scholar] [CrossRef]
- Oelschlägel, S. Characterization of unifloral honeys using HS-SPME/GC/MS. Proceedings of the 42nd APIMONDIA 2011, Buenos Aires–Argentina; Available online: http://www.apinews.com/pdf/apimondia2011/technologyquality/characterization_of_unifloral_honeys_using_hs-spme_gc_ms-stefanie_oelschlagel.pdf (accessed on 19 March 2013).
- Dias, L.A.; Peres, A.M.; Vilas-Boas, M.; Rocha, M.A.; Estevinho, L.; Machado, A.A.S.C. An electronic tongue for honey classification. Microchim. Acta 2008, 163, 97–102. [Google Scholar] [CrossRef]
- Henriques, A.; Burton, N.F.; Cooper, R.A. Antibacterial activity of selected Portuguese honeys. J. Apic. Res. 2005, 44, 119–123. [Google Scholar]
- Estevinho, L.M.; Feás, X.; Seijas, J.A.; Vázquez-Tato, M.P. Organic honey from Trás-Os-Montes region (Portugal): Chemical, palynological, microbiological and bioactive compounds characterization. Food Chem. Toxicol. 2012, 50, 258–264. [Google Scholar] [CrossRef]
- Feás, X.; Estevinho, L.M. A survey of the in vitro antifungal activity of heather (Erica sp.) organic honey. J. Med. Food 2011, 14, 1–5. [Google Scholar] [CrossRef]
- Carter, C.; Thornburg, R.W. Is the nectar redox cycle a floral defense against microbial attack? Trends Plant Sci. 2004, 9, 320–324. [Google Scholar] [CrossRef]
- Toro, M.V.; García, M.D.; Pascual, M. Isolement et identification des acides phènols chez Erica andevalensis Cabezudo-Ribera: leur contribution à l’activité antimicrobienne de l’espèce. Ann. Pharm. Fr. 1987, 45, 401–407. [Google Scholar]
- Reyes, M.; Martín-Cordero, C.; Ayuso, M.J.; Toro, M.V.; Alarcon de la Lastra, C. Antiulcer activity in rats by flavonoids of Erica andevalensis Cabezudo-Rivera. Phytother. Res. 1996, 10, 300–303. [Google Scholar] [CrossRef]
- Güvenç, A.; Kendir, G.; Eken, A. Evaluation of inorganic compounds of Erica L. species (Ericaceae) native to Turkey. FABAD J. Pharm. Sci. 2007, 32, 121–125. [Google Scholar]
- Pyrzyńska, K.; Biesaga, M. Analysis of phenolic acids and flavonoids in honey. Trends Anal. Chem. 2009, 28, 893–902. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, M.L. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Bogdanov, S. Honey as Nutrient and Functional Food: A Review. Available online: http://www.apitherapie.ch/files/files/Honig/8HoneyNutrientFunctionalReview.pdf (accessed on 11 May 2012).
- Weston, R. The contribution of catalase and other natural products to the antibacterial activity of honey: A review. Food Chem. 2000, 71, 235–239. [Google Scholar] [CrossRef]
- Kwakman, P.H.S.; Velde, A.A.; Boer, L.; Vandenbroucke-Grauls, C.M.J.E.; Zaat, S.A.J. Two Major Medicinal Honeys Have Different Mechanisms of Bactericidal Activity. PLoS One 2011, 6, e17709. [Google Scholar]
- Pappas, P.G.; Rex, J.H.; Lee, J.; Hamill, R.J.; Larsen, R.A.; Powderly, W.; Kauffman, C.A.; Hyslop, N.; Mangino, J.E.; Chapman, S.; et al. A prospective observational study of candidemia: Epidemiology, Therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin. Infect. Dis. 2003, 37, 634–643. [Google Scholar] [CrossRef]
- Perfect, J.R.; Casadevall, A. Cryptococcosis. Infect. Dis. Clin. North Am. 2002, 16, 837–874. [Google Scholar] [CrossRef]
- Molan, P.C. The antibacterial activity of honey. 1. The nature of the antibacterial activity. Bee World 1992, 73, 5–28. [Google Scholar]
- Xu, P.; Shi, M.; Chen, X.-X. Antimicrobial peptide evolution in the asiatic honey bee Apis cerana. PLoS One 2004, 4, e4239. [Google Scholar]
- Oelschlaegel, S.; Pieper, L.; Staufenbiel, R.; Gruner, M.; Zeippert, L.; Pieper, B.; Koelling-Speer, I.; Speer, K. Floral markers of cornflower (Centaurea cyanus) honey and its peroxide antibacterial activity for an alternative treatment of digital dermatitis. J. Agric. Food Chem. 2012, 60, 11811–11820. [Google Scholar] [CrossRef]
- Feás, X.; Pires, J.; Estevinho, M.L.; Iglesias, A.; Araujo, J.P.P. Palynological and physicochemical data characterisation of honeys produced in the Entre-Douro e Minho region of Portugal. Int. J. Food Sci. Tech. 2010, 45, 1255–1262. [Google Scholar] [CrossRef]
- Iglesias, A.; Feás, X.; Rodrigues, S.; Seijas, J.A.; Vázquez-Tato, M.P.; Dias, L.G.; Estevinho, L.M. Comprehensive study of honey with protected denomination of origin and contribution to the enhancement of legal specifications. Molecules 2012, 17, 8561–8577. [Google Scholar] [CrossRef]
- Morais, M.; Moreira, L.; Feás, X.; Estevinho, L.M. Honeybee-collected pollen from five Portuguese Natural Parks: Palynological origin, Phenolic content, Antioxidant properties and antimicrobial activity. Food Chem. Toxicol. 2011, 49, 1096–1101. [Google Scholar] [CrossRef]
- Sample Availability: Samples of honey are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Feás, X.; Iglesias, A.; Rodrigues, S.; Estevinho, L.M. Effect of Erica sp. Honey against Microorganisms of Clinical Importance: Study of the Factors Underlying this Biological Activity. Molecules 2013, 18, 4233-4246. https://doi.org/10.3390/molecules18044233
Feás X, Iglesias A, Rodrigues S, Estevinho LM. Effect of Erica sp. Honey against Microorganisms of Clinical Importance: Study of the Factors Underlying this Biological Activity. Molecules. 2013; 18(4):4233-4246. https://doi.org/10.3390/molecules18044233
Chicago/Turabian StyleFeás, Xesus, Antonio Iglesias, Sandra Rodrigues, and Leticia M. Estevinho. 2013. "Effect of Erica sp. Honey against Microorganisms of Clinical Importance: Study of the Factors Underlying this Biological Activity" Molecules 18, no. 4: 4233-4246. https://doi.org/10.3390/molecules18044233







