Triacylglyceride, Antioxidant and Antimicrobial Features of Virgin Camellia oleifera, C. reticulata and C. sasanqua Oils
Abstract
:1. Introduction
2. Results and Discussion
2.1. Triacylglycerol Profile
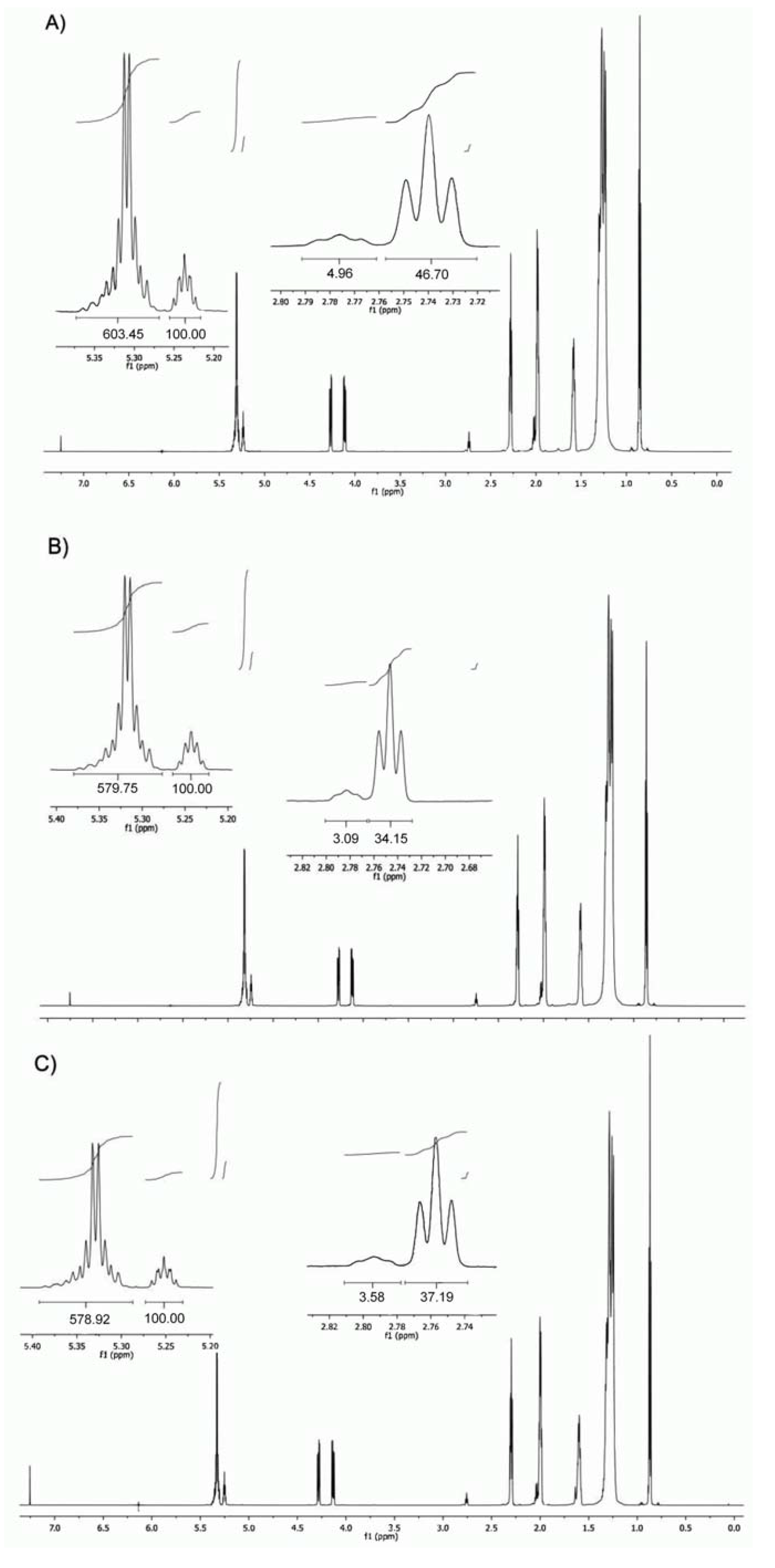
| Oil | C18:1n9c+t | C18:2n6c | C18:3n3 | ∑SFA | ∑MUFA | ∑PUFA | ∑TUFA | ω6/ω3 | ω3/ω6 | PUFA/SFA | TUFA/SFA | SFA/TUFA | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Virgin C. oleifera | 83.77 | 7.78 | 0.41 | 8.04 | 83.77 | 8.19 | 91.96 | 0.05 | 18.98 | 1.02 | 11.44 | 0.09 | PW * |
| Virgin C. reticulata | 84.47 | 5.69 | 0.26 | 9.58 | 84.47 | 5.95 | 90.42 | 0.05 | 21.88 | 0.62 | 9.44 | 0.11 | PW * |
| Virgin C. sasanqua | 82.3 | 6.2 | 0.3 | 11.2 | 82.30 | 6.5 | 88.80 | 0.05 | 20.67 | 0.58 | 7.93 | 0.13 | PW * |
| C. japonica | 80.67 | 6.65 | 0.29 | 10.0 | 80.67 | 6.94 | 87.61 | 0.04 | 22.93 | 0.69 | 8.76 | 0.11 | [21] |
| Virgin olive | 80 | 5.9 | 0.7 | 13.4 | 80.00 | 6.60 | 86.60 | 0.12 | 8.43 | 0.49 | 6.46 | 0.15 | [22] |
| Olive | 77.5 | 7.4 | 0.7 | 14.4 | 77.50 | 8.10 | 85.60 | 0.09 | 10.57 | 0.56 | 5.94 | 0.17 | [22] |
| Hazelnut | 81 | 10.7 | nd | 8.3 | 81.00 | 10.70 | 91.70 | 0.00 | - | 1.29 | 11.05 | 0.09 | [22] |
| Corn | 33 | 51 | 0.7 | 15.3 | 33.00 | 51.70 | 84.70 | 0.01 | 72.86 | 3.38 | 5.54 | 0.18 | [22] |
| Sunflower | 29.2 | 58.8 | nd | 12 | 29.20 | 58.80 | 88.00 | 0.00 | - | 4.90 | 7.33 | 0.14 | [22] |
| Linseed | 20 | 17.1 | 54.2 | 8.7 | 20.00 | 71.30 | 91.30 | 3.17 | 0.32 | 8.20 | 10.49 | 0.10 | [22] |
| Avocado | 65 | 10 | 1 | 20 | 65.00 | 11.00 | 76.00 | 0.10 | 10.00 | 0.55 | 3.80 | 0.26 | [23] |
| Tea seed | 80 | 10 | <1 | 10 | 80.00 | - | - | - | - | - | - | - | [23] |
| Pumpkin | 40.0 | 40.0 | <1 | 10.0 | 40.00 | - | - | - | - | - | - | - | [23] |
| Soybean | 25.0 | 50.0 | 7.0 | 15.0 | 25.00 | 57.00 | 82.00 | 0.14 | 7.14 | 3.80 | 5.47 | 0.18 | [23] |
| Canola | 60.0 | 20.0 | 10.0 | 7.0 | 60.00 | 30.00 | 90.00 | 0.50 | 2.00 | 4.29 | 12.86 | 0.08 | [23] |
2.2. Antioxidant Activity
| Test | C. oleifera | C.reticulata | C.sasanqua | Control |
|---|---|---|---|---|
| DPPH scavenging | 35.20 ± 4.95 ab | 33.48 ± 7.65 a | 54.87 ± 8.78 c | 47.02 ± 2.98 bc |
| Reducing power | 3.09 ± 0.92 a | 2.81 ± 0.63 a | 5.32 ± 0.98 b | 30.11 ± 1.67 c |
| LPO inhibition | 0.52 ± 0.01 a | 0.37 ± 0.01 a | 0.75 ± 0.02 b | 3.24 ± 0.56 c |
- -
- DPPH scavenging activity (EC50) : CR (33.48) < CO (35.20) < CS (54.87)
- -
- Reducing power (EC50): CR (2.81) < CO (3.09) < CS (5.32)
- -
- LPO inhibition (IC50): CR (0.37) < CO (0.52) < CS (0.75)
2.3. Antimicrobial Activity
| MIC | C. oleifera | C. reticulata | C. sasanqua | Control |
|---|---|---|---|---|
| B. cereus (ESA 239) | 52.083 ± 18.042 b | 41.667 ± 18.042 b | 104.167 ± 36.084 c | 5.08 ± 0.35 a 1 |
| C. albicans (ESA 567) | 20.833 ± 7.217 ab | 20.833 ± 7.217 ab | 29.167 ± 19.094 b | 0.65 ± 0.56 a 2 |
| E. coli (ESA 34) | 3.917 ± 3.406 ab | 2.600 ± 1.125 ab | 5.883 ± 3.406 b | 4.22 ± 1.32 a 1 |
3. Experimental
3.1. Chemicals and Reagents
3.2. Apparatus
3.3. Plant Material and Oil Extraction
3.4. 1H-NMR Analysis
3.5. Antioxidant Activity
| Signal | Functional group | Multiplicity | Chemical shift (ppm) |
|---|---|---|---|
| 1 | I (t) –CH3 | t | 0.89–0.86 |
| 2 | H (m) –CH2- | m | 1.35–1.23 |
| 3 | G (m) –CH2–C–CO2– | m | 1.64–1.57 |
| 4 | D (m) –CH2–CO2- | m | 2.02–1.98 |
| 5 | E (m) –CH2–CO2– | m | 2.06–2.02 |
| 6 | F (dt) –C–CH2–C=C– | dt | 2.33–2.28 |
| 7 | C (t) –C=C–CH2–C=C– | t | 2.78–2.74 |
| 8 | L (m) –C=C–CH2–C=C–CH2–C=C | m | 2.81–2.78 |
| 9 | A (dd) –C–CH2–O–CO–C | dd | 4.15–4.06 |
| 10 | B (dd) –C–CH2–O–CO–C | dd | 4.30–4.26 |
| 11 | K (m) CH(–C–O–CO–C–)2 | m | 5.27–5.24 |
| 12 | J (m) C–HC=CH–C | m | 5.37–5.30 |
3.5.1. DPPH Scavenging Activity
3.5.2. Reducing Power Assay
3.5.3. β-Carotene Bleaching (BCB) Assay
3.6. Antimicrobial Activity Tests
3.6.1. Microbial Strains
3.6.2. Growth Conditions
3.6.3. Antimicrobial Assay
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Ming, T.L.; Bartholomew, B. Theaceae. In Flora of China; Wu, Z.Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China; Missouri Botanical Garden: St. Louis, MO, USA, 2007; Volume 12, pp. 366–478. [Google Scholar]
- Jung, E.; Lee, J.; Baek, J.; Jung, K.; Lee, J.; Huha, S.; Kim, S.; Koh, J.; Park, D. Effect of Camellia japonica oil on human type I procollagen production and skin barrier function. J. Ethnopharmacol. 2007, 112, 127–131. [Google Scholar] [CrossRef]
- Kim, K.Y.; Davidson, P.M.; Chung, H.J. Antibacterial activity in extracts of Camellia Japonica L. petals and its application to a model food system. J. Food Protect. 2001, 64, 1255–1260. [Google Scholar]
- Onodera, K.; Hanashiro, K.; Yasumoto, T. Camellianoside, a novel antioxidant glycoside from the leaves of Camellia japonica. Biosci. Biotechnol. Biochem. 2006, 70, 1995–1998. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Chew, Y.L. Antioxidant activity of Camellia sinensis leaves and tea from a low land plantation in Malaysia. Food Chem. 2007, 102, 1214–1222. [Google Scholar] [CrossRef]
- Onodera, K.I.; Tsuha, K.; Yasumoto-Hirose, M.; Tsuha, K.; Hanashiro, K.; Naoki, H.; Yasumoto, T. Okicamelliaside, an extraordinarily potent anti-degranulation glucoside isolated from leaves of Camellia japonica. Biosci. Biotechnol. Biochem. 2010, 74, 2532–2534. [Google Scholar] [CrossRef]
- Kuba, M.; Tsuha, K.; Tsuha, K.; Matsuzaki, G.; Yasumoto, T. In vivo analysis of the anti-allergic activities of Camellia japonica extract and okicamelliaside, a degranulation inhibitor. J. Health Sci. 2008, 54, 584–588. [Google Scholar] [CrossRef]
- Park, J.C.; Hur, J.M.; Park, J.G.; Hatano, T.; Yoshida, T.; Miyashiro, H.; Min, B.S.; Hattori, M. Inhibitory effects of Korean medicinal plants and camelliatannin H from Camellia japonica on human immunodeficiency virus type 1 protease. Phytother. Res. 2002, 16, 422–426. [Google Scholar] [CrossRef]
- Akihisa, T.; Tokuda, H.; Ukiya, M.; Suzuki, T.; Enjo, F.; Koike, K.; Nikaido, T.; Nishino, H. 3-Epicabraleahydroxylactone and other triterpenoids from Camellia oil and their inhibitory effects on Epstein-Barr virus activation. Chem. Pharm. Bull. 2004, 52, 153–156. [Google Scholar] [CrossRef]
- Ma, J.; Ye, H.; Rui, Y.; Chen, G.; Zhang, N. Fatty acid composition of Camellia oleifera oil. J. Verbr. Lebensm. 2011, 6, 9–12. [Google Scholar] [CrossRef]
- Zhang, L.L.; Wang, Y.M.; Wu, D.M.; Xu, M.; Chen, J.H. Comparisons of antioxidant activity and total phenolics of Camelia oleifera Abel fruit hull from different regions of China. J. Med. Plants Res. 2010, 4, 1420–1426. [Google Scholar]
- Shen, J.; Zhang, Z.; Tian, B.; Hua, Y. Lipophilic phenols partially explain differences in the antioxidant activity of subfractions from methanol extract of Camellia oil. Eur. Food Res. Technol. 2012, 235, 1071–1082. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, Y.T.; Park, J.W.; Rheel, C. Antioxidation activity of oil extracts prepared from various seeds. Food Sci. Biotechnol. 2012, 21, 637–643. [Google Scholar] [CrossRef]
- Sokol'skii, I.N.; Ban'kovskii, A.I.; Zinkevich, É.P. Triterpene glycosides from Camellia oleifera and Camellia sasanqua. Chem. Nat. Compd. 1975, 11, 116–117. [Google Scholar] [CrossRef]
- Li, H.; Zhou, G.-Y.; Zhang, H.-Y.; Liu, J.-A. Research progress on the health function of tea oil. J. Med. Plants Res. 2011, 5, 485–489. [Google Scholar]
- Wang, L.; Lee, F.S.C.; Wang, X.; He, Y. Feasibility study of quantifying and discriminating soybean oil adulteration in Camellia oils by attenuated total reflectance MIR and fiber optic diffuse reflectance NIR. Food Chem. 2006, 95, 529–536. [Google Scholar] [CrossRef]
- Zeb, A. Triacylglycerols composition, oxidation and oxidation compounds in camellia oil using liquid chromatography-mass spectrometry. Chem. Phys. Lipids 2012, 165, 608–614. [Google Scholar] [CrossRef]
- Wenk, M.R. The emerging field of lipidomics. Nat. Rev. Drug Discov. 2005, 4, 594–610. [Google Scholar] [CrossRef]
- Dais, P.; Spyros, A.; Christophoridou, S.; Hatzakis, E.; Fragaki, G.; Agiomyrgianaki, A.; Salivaras, E.; Siragakis, G.; Daskalaki, D.; Tasiolula-Margari, M.; Brenes, M. Comparison of Analytical Methodologies Based on 1H and 31P-NMR Spectroscopy with Conventional Methods of Analysis for the Determination of Some Olive Oil Constituents. J. Agric. Food Chem. 2007, 55, 577–584. [Google Scholar] [CrossRef]
- Jie, M.S.; Mustafa, J. High-resolution nuclear magnetic resonance spectroscopy—Applications to fatty acids and triacylglycerols. Lipids 1997, 32, 1019–1034. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Study by means of 1H nuclear magnetic resonance of the oxidation process undergone by edible oils of different natures submitted to microwave action. Food Chem. 2006, 96, 665–674. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. High resolution 1H nuclear magnetic resonance in the study of edible oils and fats. Trends Food Sci. Technol. 2001, 12, 328–338. [Google Scholar] [CrossRef]
- Haiyan, Z.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Endogenous biophenol, fatty acid and volatile profiles of selected oils. Food Chem. 2006, 100, 1544–1551. [Google Scholar]
- Vlahov, G. Application of NMR to the study of olive oils. Prog. Nucl. Magn. Reson. Spectrosc. 1999, 35, 341–357. [Google Scholar] [CrossRef]
- Barison, A.; da Silva, C.W.; Campos, F.R.; Simonelli, F.; Lenz, C.A.; Ferreira, A.G. A simple methodology for the determination of fatty acid composition in edible oils through 1H NMR spectroscopy. Magn. Res. Chem. 2010, 48, 642–650. [Google Scholar]
- Salinero, C.; Feás, X.; Mansilla, J.P.; Seijas, J.A.; Vázquez-Tato, M.P.; Vela, P.; Sainz, M.J. 1H-nuclear magnetic resonance analysis of the triacylglyceride composition of cold-pressed oil from Camellia japonica. Molecules 2012, 17, 6716–6727. [Google Scholar] [CrossRef]
- Escrich, E.; Moral, R.; Grau, L.; Costa, I.; Solanas, M. Molecular mechanisms of the effects of olive oil and other dietary lipids on cancer. Mol. Nutr. Food Res. 2007, 51, 1279–1292. [Google Scholar]
- Kaya-Dagistanli, F.; Tanriverdi, G.; Altinok, A.; Ozyazgan, S.; Ozturk, M. The effects of alpha lipoic acid on liver cells damages and apoptosis induced by polyunsaturated fatty acids. Food Chem. Toxicol. 2013, 53, 84–93. [Google Scholar] [CrossRef]
- Pérez-Jiménez, F.; Ruano, J.; Pérez-Martinez, P.; López-Segura, F.; López-Miranda, J. The influence of olive oil on human health: Not a question of fat alone. Mol. Nutr. Food Res. 2007, 51, 1199–1208. [Google Scholar]
- Castelo-Branco, V.N.; Torres, A.G. Generalized linear model describes determinants of total antioxidant capacity of refined vegetable oils. Eur. J. Lipid Sci. Tech. 2012, 114, 332–342. [Google Scholar] [CrossRef]
- Pinchuk, I.; Shoval, H.; Dotan, Y.; Lichtenberg, D. Evaluation of antioxidants: Scope, limitations and relevance of assays. Chem. Phys. Lipids 2012, 165, 638–647. [Google Scholar] [CrossRef]
- Lee, C.P.; Yen, G.C. Antioxidant activity and bioactive compounds of tea seed (Camellia oleifera Abel.) oil. J. Agric. Food Chem. 2006, 54, 779–784. [Google Scholar] [CrossRef]
- Zhang, Z.Y. Study on the Radiation Activity of Antioxidant Ingredients and Mechanism of Tea Oil; Zhejiang University Press: Hangzhou ,China, 2006; pp. 1–36. [Google Scholar]
- Tenover, F.C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Med. 2006, 119, S3–S10. [Google Scholar] [CrossRef]
- Livermore, D.M. Bacterial resistance: Origins, epidemiology, and impact. Clin. Infect. Dis. 2003, 15, 11–23. [Google Scholar] [CrossRef]
- Kotiranta, A.; Lounatmaa, K.; Haapasalo, M. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2000, 2, 189–198. [Google Scholar] [CrossRef]
- Bottone, E.J. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 2010, 23, 382–398. [Google Scholar] [CrossRef]
- Fridkin, S.K. The changing face of fungal infections in health care settings. Clin. Infect. Dis. 2005, 41, 1455–1460. [Google Scholar] [CrossRef]
- Tortorano, A.M.; Kibbler, C.; Peman, J.; Bernhardt, H.; Klingspor, L.; Grillot, R. Candidaemia in Europe: Epidemiology and resistance. Int. J. Antimicrob. Agents 2006, 27, 359–366. [Google Scholar] [CrossRef]
- Croxen, M.A.; Finlay, B.B. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 2010, 8, 26–38. [Google Scholar]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Tadesse, D.A.; Zhao, S.; Tong, E.; Ayers, S.; Singh, A.; Bartholomew, M.J.; McDermott, P.F. Antimicrobial drug resistance in Escherichia coli from humans and food animals, United States, 1950-2002. Emerg. Infect. Dis. 2012, 18, 741–749. [Google Scholar] [CrossRef]
- Hu, J.L.; Nie, S.P.; Huang, D.F.; Li, C.; Xie, M.Y. Extraction of saponin from Camellia oleifera cake and evaluation of its antioxidant activity. Int. J. Food Sci. Tech. 2012, 47, 1676–1687. [Google Scholar] [CrossRef]
- Hu, J.L.; Nie, S.P.; Huang, D.F.; Li, C.; Xie, M.Y.; Wan, Y. Antimicrobial activity of saponin-rich fraction from Camellia oleifera cake and its effect on cell viability of mouse macrophage RAW 264.7. J. Sci. Food Agric. 2012, 92, 2443–2449. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B.; Bautista, D.A. Antibacterial activity of green tea polyphenols against Escherichia coli K12. Nahrung 2000, 44, 60–62. [Google Scholar] [CrossRef]
- Hassan, S.M.; Haq, A.U.; Byrd, J.A.; Berhow, M.A.; Cartwright, A.L.; Bailey, C.A. Haemolytic and antimicrobial activities of saponin-rich extracts from guar meal. Food Chem. 2010, 119, 600–605. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz-Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Vela, P.; Salinero, C.; Sainz, M.J. Phenological growth stages of Camellia japonica. Ann. Appl. Biol. 2013, 162, 182–190. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Moreira, L.; Dias, L.G.; Pereira, J.A.; Estevinho, L. Antioxidant properties, total phenols and pollen analysis of propolis samples from Portugal. Food Chem. Toxicol. 2008, 46, 3482–3485. [Google Scholar] [CrossRef]
- Morais, M.; Moreira, L.; Feás, X.; Estevinho, L.M. Honeybee-collected pollen from five Portuguese Natural Parks: Palynological origin, phenolic content, antioxidant properties and antimicrobial activity. Food Chem. Toxicol. 2011, 49, 1096–1101. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the oils are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Feás, X.; Estevinho, L.M.; Salinero, C.; Vela, P.; Sainz, M.J.; Vázquez-Tato, M.P.; Seijas, J.A. Triacylglyceride, Antioxidant and Antimicrobial Features of Virgin Camellia oleifera, C. reticulata and C. sasanqua Oils. Molecules 2013, 18, 4573-4587. https://doi.org/10.3390/molecules18044573
Feás X, Estevinho LM, Salinero C, Vela P, Sainz MJ, Vázquez-Tato MP, Seijas JA. Triacylglyceride, Antioxidant and Antimicrobial Features of Virgin Camellia oleifera, C. reticulata and C. sasanqua Oils. Molecules. 2013; 18(4):4573-4587. https://doi.org/10.3390/molecules18044573
Chicago/Turabian StyleFeás, Xesús, Leticia M. Estevinho, Carmen Salinero, Pilar Vela, María J. Sainz, María Pilar Vázquez-Tato, and Julio A. Seijas. 2013. "Triacylglyceride, Antioxidant and Antimicrobial Features of Virgin Camellia oleifera, C. reticulata and C. sasanqua Oils" Molecules 18, no. 4: 4573-4587. https://doi.org/10.3390/molecules18044573







