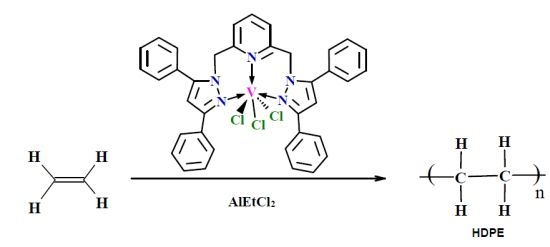
A New Vanadium (III) Complex of 2,6-Bis(3,5-diphenylpyrazol-1-ylmethyl)pyridine as a Catalyst for Ethylene Polymerization
Abstract
:1. Introduction
2. Results and Discussion
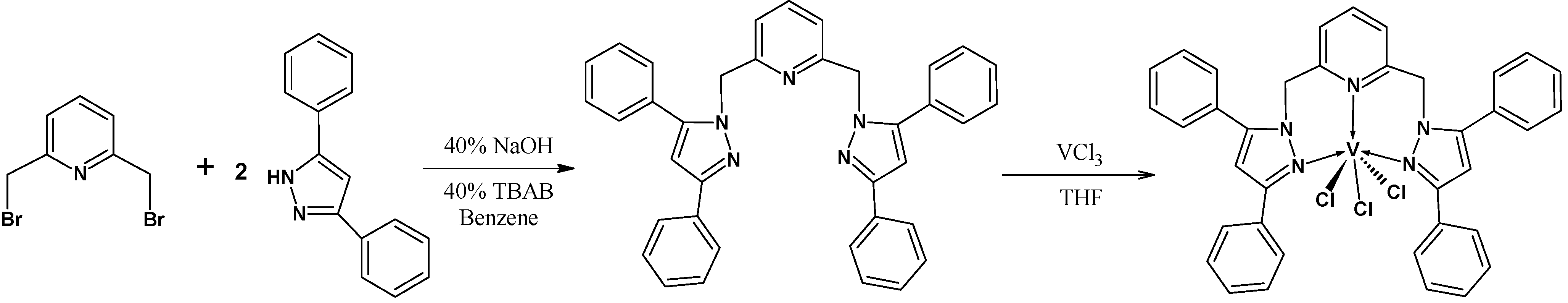
2.1. Synthesis of 2,6-Bis(3,5-diphenylpyrazol-1-ylmethyl)pyridine and Its Vanadium Complex
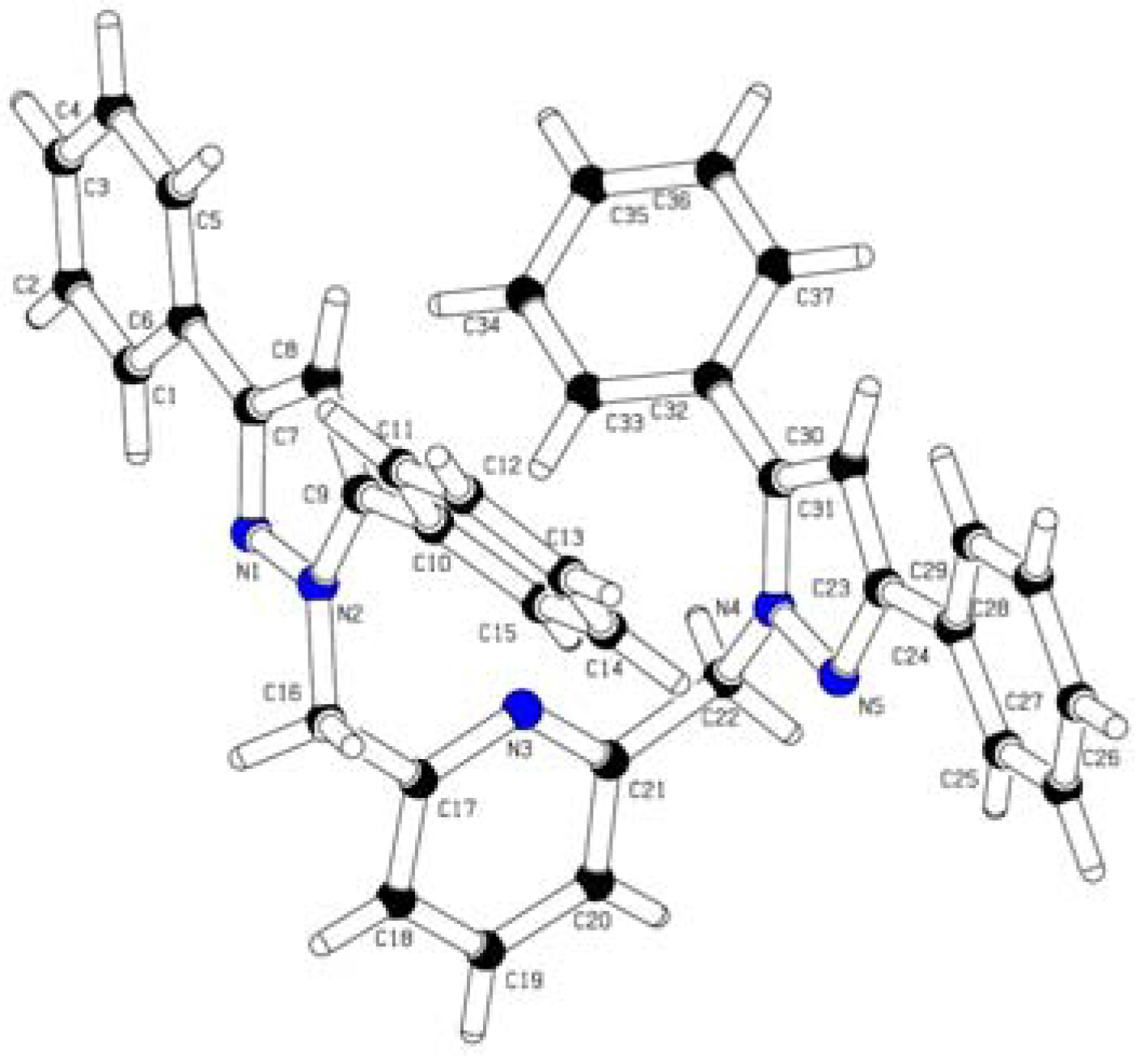
| CCDC deposition | 688192 | |
|---|---|---|
| Empirical formula | C37H29N5 | |
| Formula weight | 543.65 | |
| Temperature | 298(2) K | |
| Wavelength | 0.71073 Å | |
| Crystal system | Orthorhombic | |
| Space group | Pbca | |
| Unit cell dimensions | a = 16.5531(4) Å | α = 90°. |
| b = 9.5085(2) Å | β = 90°. | |
| c = 37.3606(8) Å | γ = 90°. | |
| Volume | 5880.4(2) Å3 | |
| Z | 8 | |
| Density (calculated) | 1.228 Mg/m3 | |
| Absorption coefficient | 0.074 mm−1 | |
| F(000) | 2288 | |
| Crystal size | 0.32 × 0.14 × 0.12 mm3 | |
| Theta range for data collection | 1.09 to 28.00°. | |
| Index ranges | −20<=h<=21, −12<=k<=11, −48<=l<=49 | |
| Reflections collected | 68614 | |
| Independent reflections | 7105 [R(int) = 0.1090] | |
| Completeness to theta = 28.00° | 100.0% | |
| Absorption correction | None | |
| Max. and min. transmission | 0.9912 and 0.9768 | |
| Refinement method | Full-matrix least-squares on F2 | |
| Data/restraints/parameters | 7105/0/380 | |
| Goodness-of-fit on F2 | 0.670 | |
| Final R indices [I>2sigma(I)] | R1 = 0.0400, wR2 = 0.0928 | |
| R indices (all data) | R1 = 0.1564, wR2 = 0.1467 | |
| Extinction coefficient | 0.0021(3) | |
| Largest diff. peak and hole | 0.229 and −0.180 e−3 Å | |
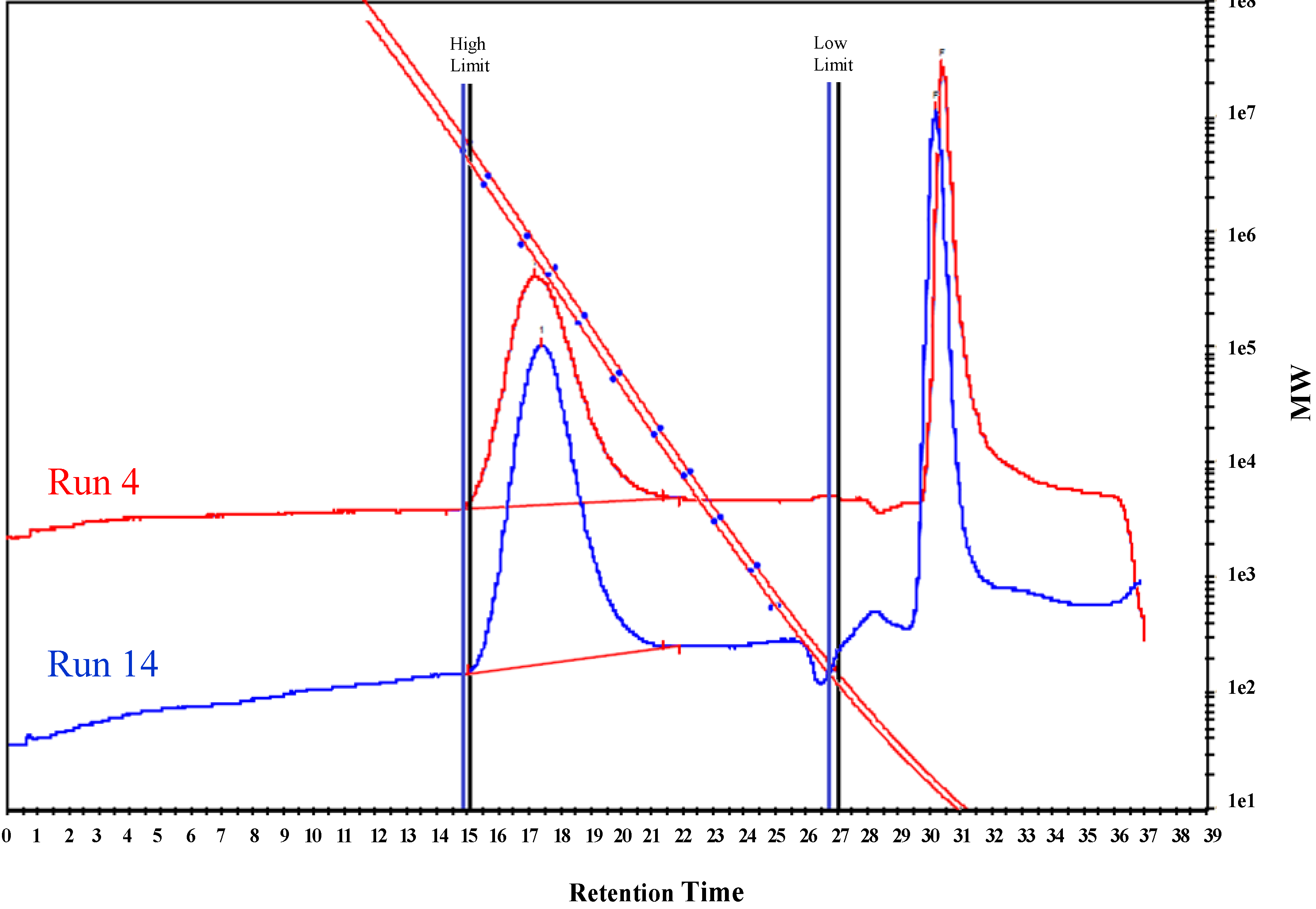
2.2. Polymerization of Ethylene
| Run No. | Cat(μmol) | Al/V ratio | Temp(°C) | Time(min) | P C2H4(bar) | YieldPE/g | Activity(Kg mol−1·h−1) | Mw×10−6 | PD |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1(2.5) | 1500 | 25 | 30 | 10 | 1.25 | 1000 | 0.89 | 2.7 |
| 2 | 1(2.5) | 1500 | 50 | 30 | 10 | 0.38 | 304 | 0.73 | 4.3 |
| 3 | 1(1.5) | 1500 | 25 | 15 | 10 | 0.59 | 1570 | 1.0 | 3.1 |
| 4 | 1(1.5) | 1500 | 25 | 30 | 10 | 0.99 | 1320 | 0.92 | 2.8 |
| 5 | 1(5) | 1500 | 25 | 60 | 10 | 1.27 | 270 | -- | -- |
| 6 | 1(1.5) | 1000 | 25 | 30 | 10 | 0.47 | 626 | -- | -- |
| 7 | 1(1.5) | 2000 | 25 | 15 | 10 | 0.21 | 560 | -- | -- |
| 8 | 1(2.5) | 1500 | 25 | 15 | 1 | 0.17 | 272 | 0.74 | 4.3 |
| 9 | 2(5) | 500 | 25 | 10 | 10 | 2.0 | 2400 | 0.6 | 2.9 |
| 10 | 2(5) | 500 | 25 | 60 | 10 | 4.3 | 860 | --- | --- |
| 11 | 2(5) | 250 | 25 | 10 | 10 | 2.5 | 3010 | 0.8 | 2.2 |
| 12 | 2(5) | 250 | 25 | 30 | 10 | 3.1 | 1240 | -- | --- |
| 13 | 2(5) | 250 | 50 | 30 | 10 | 0.73 | 292 | -- | -- |
| 14 | 2(5) | 250 | 25 | 60 | 10 | 4.7 | 940 | 0.68 | 2.5 |
| 15 | 2(2.5) | 500 | 25 | 30 | 10 | 1.7 | 1360 | -- | --- |
| 16 | 2(2.5) | 500 | 25 | 60 | 10 | 3.5 | 1400 | 0.58 | 2.4 |
| 17 | 2(5) | 1000 | 25 | 60 | 10 | 4.2 | 840 | 0.59 | 2.3 |
| 18 | 2(5) | 1000 | 25 | 120 | 10 | 5.6 | 560 | -- | -- |
| 19 | 2(5) | 250 | 25 | 10 | 1 | 0.35 | 422 | 0.75 | 3.2 |
| 20 | 2(5) | 250 | 25 | 60 | 1 | 2.1 | 410 | 0.6 | 2.5 |
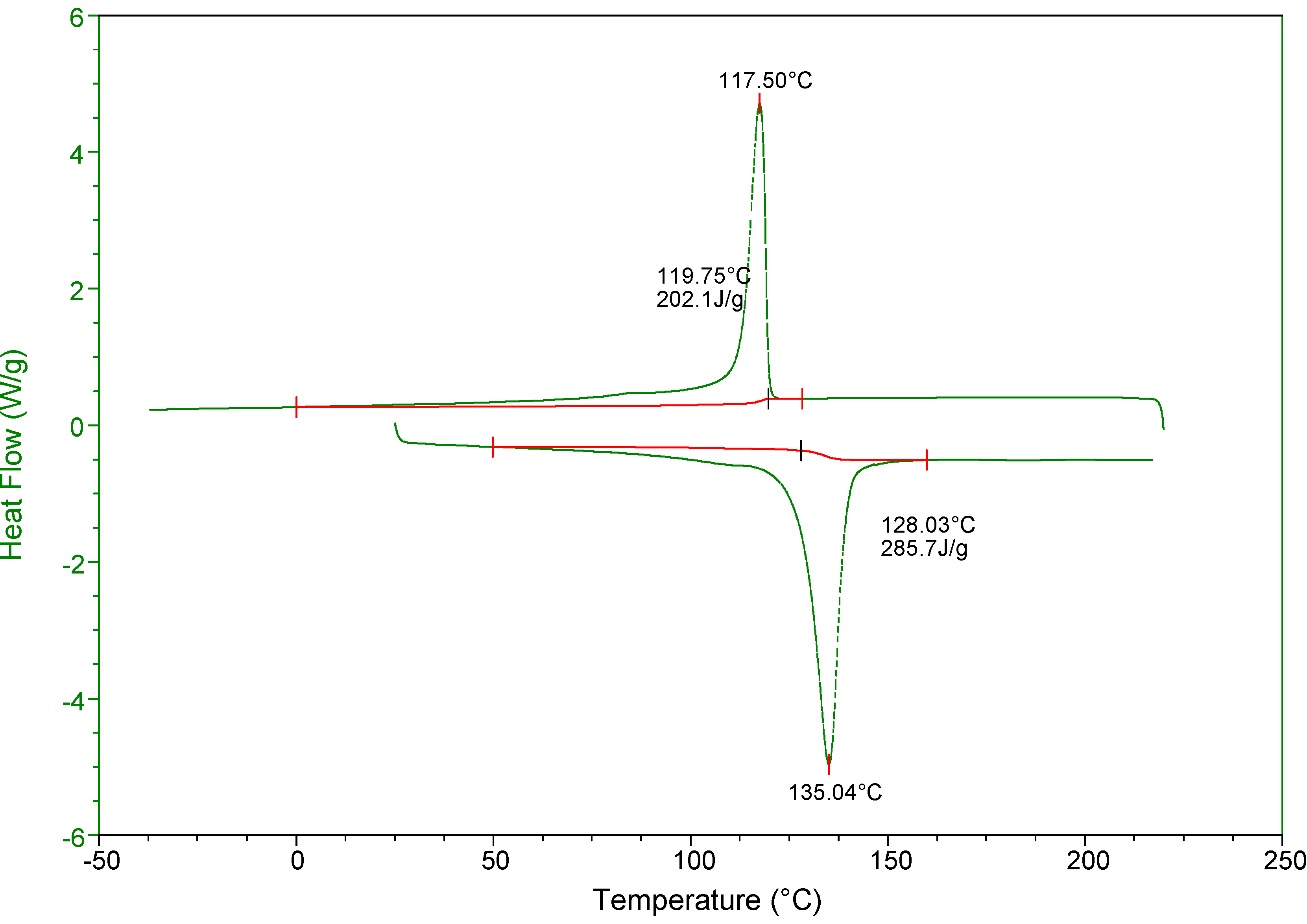
3. Experimental
3.1. General
3.2. Synthesis of 3,5-Diphenylpyrazole
3.3. Synthesis of 2,6-[(3,5-ph2pz-CH2)2-py]
3.4. Synthesis of {2,6-[(3,5-ph2pz)CH2]2py}VCl3 (1)
4. Conclusions
Supplementary Materials
Acknowledgments
References
- Constable, E.C.; Steel, P.J. N,N'-chelating biheteroaromatic ligands; a survey. Coord. Chem. Rev. 1989, 93, 205–223. [Google Scholar] [CrossRef]
- House, D.A.; Steel, P.J.; Watson, A.A. Chiral heterocyclic ligands. Part V. The first X-ray structure of an octahedral transition metal complex containing a strongly chelating bidentate perchlorate. J. Chem. Soc. Chem. Commun. 1987, 1575–1576. [Google Scholar] [CrossRef]
- Driessen, W.L.; De Graaff, R.A.G.; Wiesmeijer, W.G.R. The structure of the mixed-ligand compound [{bis(1-pyrazolylmethyl)amine}(pyrazole) (tetrafluoroborato)copper(II)] tetra-fluoroborate. Acta Crystallogr. Sect. C 1987, 43C, 2319–2321. [Google Scholar]
- Tsuji, S.; Swenson, D.C.; Jordan, R.F. Neutral and cationic palladium(II) bis(pyrazolyl)methane complexes. Orgnometallics 1999, 18, 4758–4764. [Google Scholar] [CrossRef]
- Karam, A.R.; Catari, E.L.; Lopez-Linares, F.; Agrifoglio, G.; Albano, C.L.; Diaz-Barrios, A.; Lehmann, T.E.; Pekerar, S.V.; Albornoz, L.A.; Atencio, R.; et al. Synthesis, characterization and olefin polymerization studies of iron(II) and cobalt(II) catalysts bearing 2,6-bis(pyrazol-1-yl)pyridines and 2,6-bis(pyrazol-1-ylmethyl) pyridines ligand. Appl. Catal. A Gen. 2005, 280, 165–173. [Google Scholar] [CrossRef]
- Abbo, H.S.; Mapolie, S.F.; Darkwa, J.; Titinchi, S.J.J. Bis(pyrazolyl)pyridine vanadium(III) complexes as highly active ethylene polymerization catalysts. J. Organomet. Chem. 2007, 692, 5327–5330. [Google Scholar] [CrossRef]
- Zabel, D.; Schubert, A.; Wolmershaeuser, G.; Jones, R.L., Jr.; Thiel, W.R. Iron and cobalt complexes of tridentate N-donor ligands in ethylene polymerization: Efficient shielding of the active sites by simple phenyl groups. Eur. J. Inorg. Chem. 2008, 3648–3654. [Google Scholar]
- Ojwach, S.O.; Guzei, I.A.; Benade, L.L.; Mapolie, S.F.; Darkwa, J. Pyrazol-l-ylmethyl)pyridine nickel complexes: Ethylene oligomerization and unusual Friedel - Crafts alkylation catalysts. Organometallics 2009, 28, 2127–2133. [Google Scholar] [CrossRef]
- Abbo, H.S.; Titinchi, S.J.J. Bis(pyrazolyl)pyridine late transition metal complexes as single-site catalysts for ethylene polymerization to highly linear polyethylene. Catal. Lett. 2010, 139, 90–96. [Google Scholar] [CrossRef]
- Malcolm, A. The synthesis and coordination chemistry of 2,6-bis(pyrazolyl) pyridines and related ligands − Versatile terpyridine analogues. Coord. Chem. Rev. 2005, 249, 2880–2908. [Google Scholar] [CrossRef]
- Mukherjee, R. Coordination chemistry with pyrazole-based chelating ligands: Molecular structural aspects. Coord. Chem. Rev. 2000, 203, 151–218. [Google Scholar] [CrossRef]
- Halcrow, M.A. Iron(II) complexes of 2,6-di(pyrazol-1-yl)pyridines-A versatile system for spin-crossover research. Coord. Chem. Rev. 2009, 253, 2493–2514. [Google Scholar] [CrossRef]
- Pons, J.; Chadghan, A.; García-Antón, J.; Ros, J. Phenyl and pyridyl bis-pyrazoles: Synthesis from the bis(β-diketone) precursors and characterization by analytical and spectroscopic methods. Lett. Org. Chem. 2010, 7, 178–181. [Google Scholar] [CrossRef]
- Montoya, V.; Pons, J.; Branchadell, V.; Garcia-Antón, J.; Font-Bardía, M.; Ros, J. Highly efficient pyridylpyrazole ligands for the heck reaction. A combined experimental and computational study. Organometallics 2008, 27, 1084–1091. [Google Scholar]
- Gibson, V.C.; Redshaw, C.; Solan, G.A. Bis(imino)pyridines: Surprisingly reactive ligands and a gateway to new families of catalysts. Chem. Rev. 2007, 107, 1745–1776. [Google Scholar] [CrossRef]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-metal catalysts for ethylene homo- and copolymerization. Chem. Rev. 2000, 100, 1169–1203. [Google Scholar] [CrossRef]
- Von Dreele, R.B.; Fay, R.C. Octahedral vanadium(IV) complexes. Synthesis and stereochemistry of vanadium(IV) .beta.-diketonates. J. Am. Chem. Soc. 1972, 94, 7935–7936. [Google Scholar] [CrossRef]
- Reger, D.L.; Semeniuc, R.F.; Smith, M.D. Metal complexes of 2,6-bis[(pyrazol-1-yl)methyl]pyridine: The search for aryl-pyrazolyl embrace interactions as a synthon for crystal engineering. Cryst. Growth Des. 2005, 5, 1181–1190. [Google Scholar] [CrossRef]
- Reardon, D.; Conan, F.; Gambarotta, S.; Yap, G.; Wang, Q. Life and death of an active ethylene polymerization catalyst. Ligand involvement in catalyst activation and deactivation. Isolation and characterization of two unprecedented neutral and anionic vanadium(I) alkyls. J. Am. Chem. Soc. 1999, 121, 9318–9325. [Google Scholar] [CrossRef]
- Feghali, K.; Harding, D.J.; Reardon, D.; Gambarotta, S.; Yap, G.; Wang, O. Stability of metal-carbon bond versus metal reduction during ethylene polymerization promoted by a vanadium complex: The role of the aluminum cocatalyst. Organometallics 2002, 21, 968–976. [Google Scholar]
- Milione, S.; Cavallo, G.; Tedesco, C.; Grassi, A. Synthesis of α-diimine V(III) complexes and their role as ethylene polymerisation catalysts. J. Chem. Soc. Dalton Trans. 2002, 1839–1846. [Google Scholar]
- Janas, Z.; Jerzykiewicz, L.P.; Richards, R.L.; Sobota, P. Synthesis and molecular structure of vanadium(III) dithiolate complexes: A new class of alkene polymerization catalysts. Chem. Commun. 1999, 1015–1016. [Google Scholar]
- Wu, J.-Q.; Pan, L.; Hu, N.-H.; Li, Y.-S. Synthesis, structural characterization, and ethylene polymerization behavior of the vanadium(III) complexes bearing salicylaldiminato ligands. Organometallics 2008, 27, 3840–3848. [Google Scholar] [CrossRef]
- APEX2, Version 2.0-1; Bruker AXS Inc.: Madison, WI, USA, 2005.
- SAINT-NT, Version 6.0. (includes XPREP and SADABS); Bruker AXS Inc.: Madison, WI, USA, 2005.
- SHELXTL, Version 5.1. (includes XS, XL, XP, XSHELL); Bruker AXS Inc.: Madison, WI, USA, 1999.
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for windows - a version of ORTEP-III with a graphical user interface (GUI). J. Appl. Cryst. 1997, 30, 565. [Google Scholar] [CrossRef]
- Lipp, M.; Dallacker, F.; Munnes, S. Preparation of 3,5-diarylpyrazoles and 3,7-diaryl-4,5-dihydro-1,2-diazepines. Justus Liebigs Ann. Chem. 1958, 618, 110–117. [Google Scholar] [CrossRef]
- Sample Availability: Contact the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Abbo, H.S.; Titinchi, S.J.J. A New Vanadium (III) Complex of 2,6-Bis(3,5-diphenylpyrazol-1-ylmethyl)pyridine as a Catalyst for Ethylene Polymerization. Molecules 2013, 18, 4728-4738. https://doi.org/10.3390/molecules18044728
Abbo HS, Titinchi SJJ. A New Vanadium (III) Complex of 2,6-Bis(3,5-diphenylpyrazol-1-ylmethyl)pyridine as a Catalyst for Ethylene Polymerization. Molecules. 2013; 18(4):4728-4738. https://doi.org/10.3390/molecules18044728
Chicago/Turabian StyleAbbo, Hanna S., and Salam J. J. Titinchi. 2013. "A New Vanadium (III) Complex of 2,6-Bis(3,5-diphenylpyrazol-1-ylmethyl)pyridine as a Catalyst for Ethylene Polymerization" Molecules 18, no. 4: 4728-4738. https://doi.org/10.3390/molecules18044728