Anti-Oxidative and Anti-Proliferative Activity on Human Prostate Cancer Cells Lines of the Phenolic Compounds from Corylopsis coreana Uyeki
Abstract
:1. Introduction
2. Results and Discussion
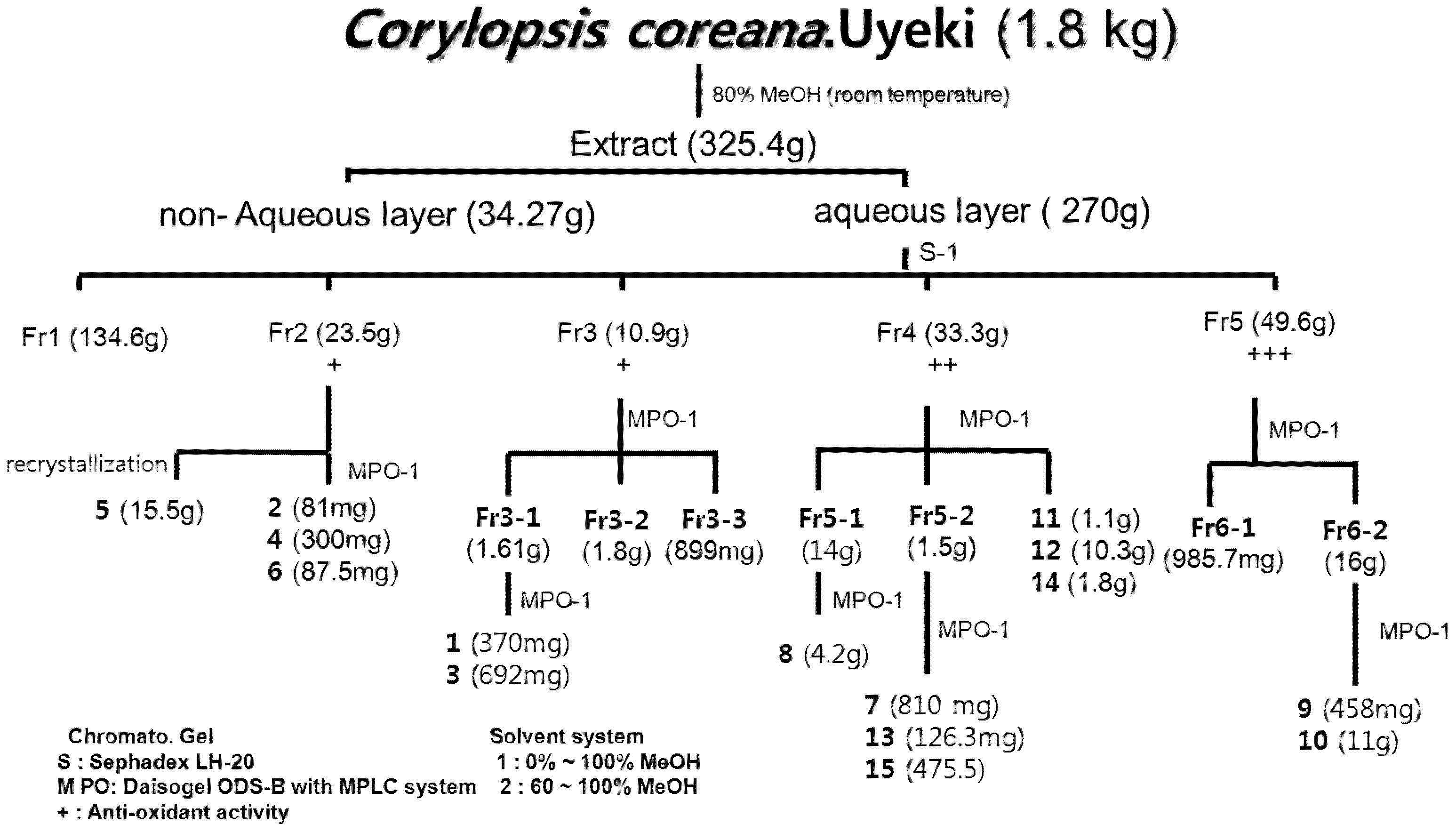
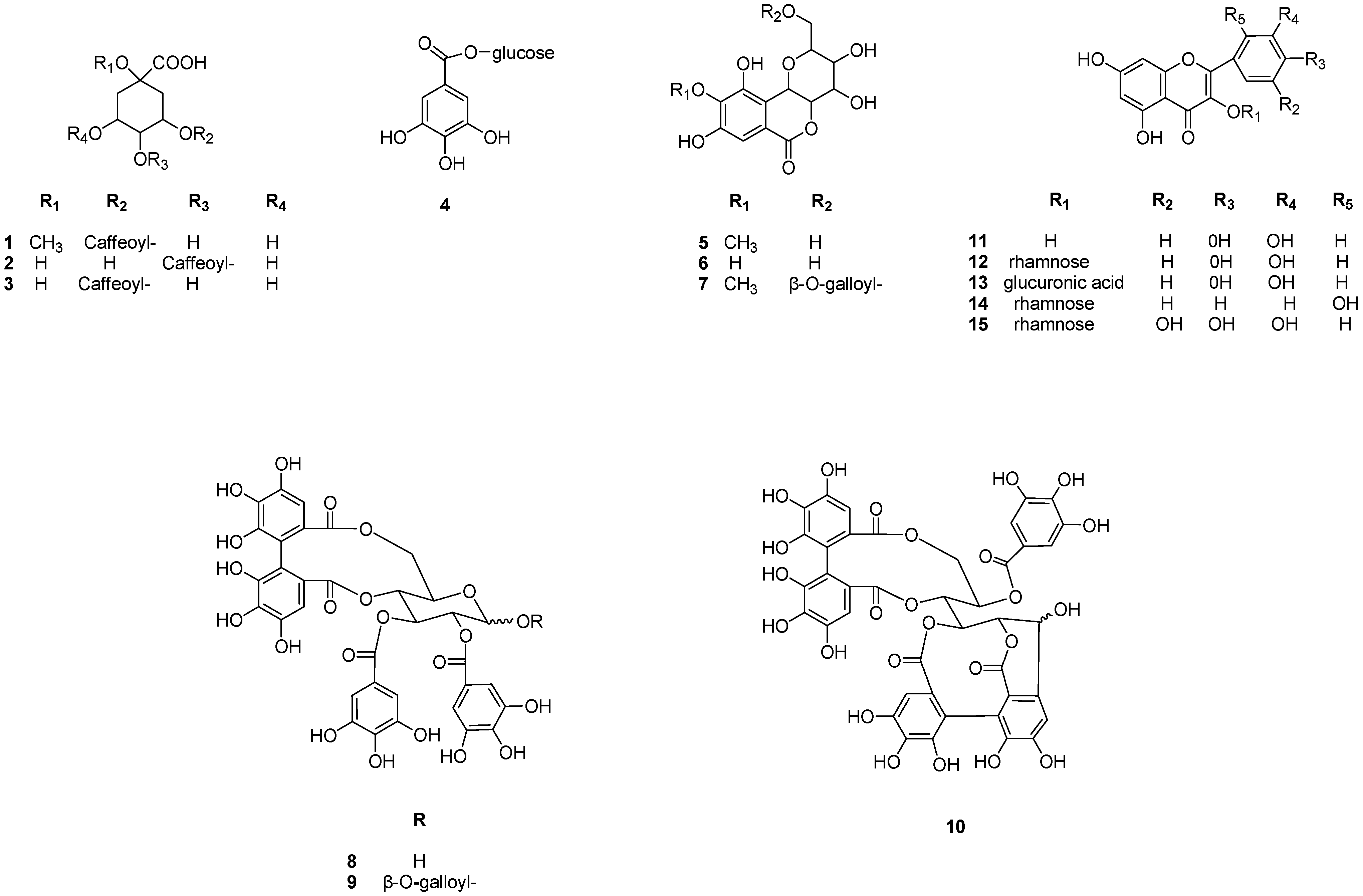
2.1. Isolation and Identification
| Fractions | DPPH radical scavenging activity SC50 (mg/mL) | Superoxide scavenging activity SC50 (mg/mL) |
|---|---|---|
| 1 | >100 e | 59.02 ± 1.07 d |
| 2 | 43.16 ± 0.48 d | 23.64 ± 0.92 c |
| 3 | 20.26 ± 0.43 c | 13.35 ± 1.09 b |
| 4 | 11.22 ±0 24 b | 5.89 ± 0.91 a |
| 5 | 5.89 ± 0.03 a | 2.42 ± 0.73 a |
| Ascorbic acid | 6.68 ± 0.11 a | - |
| Allopurinol | - | 1.70 ± 0.92 a |
2.2. Anti-Oxidative Activity (DPPH Radical, Superoxide Scavenging Activity)
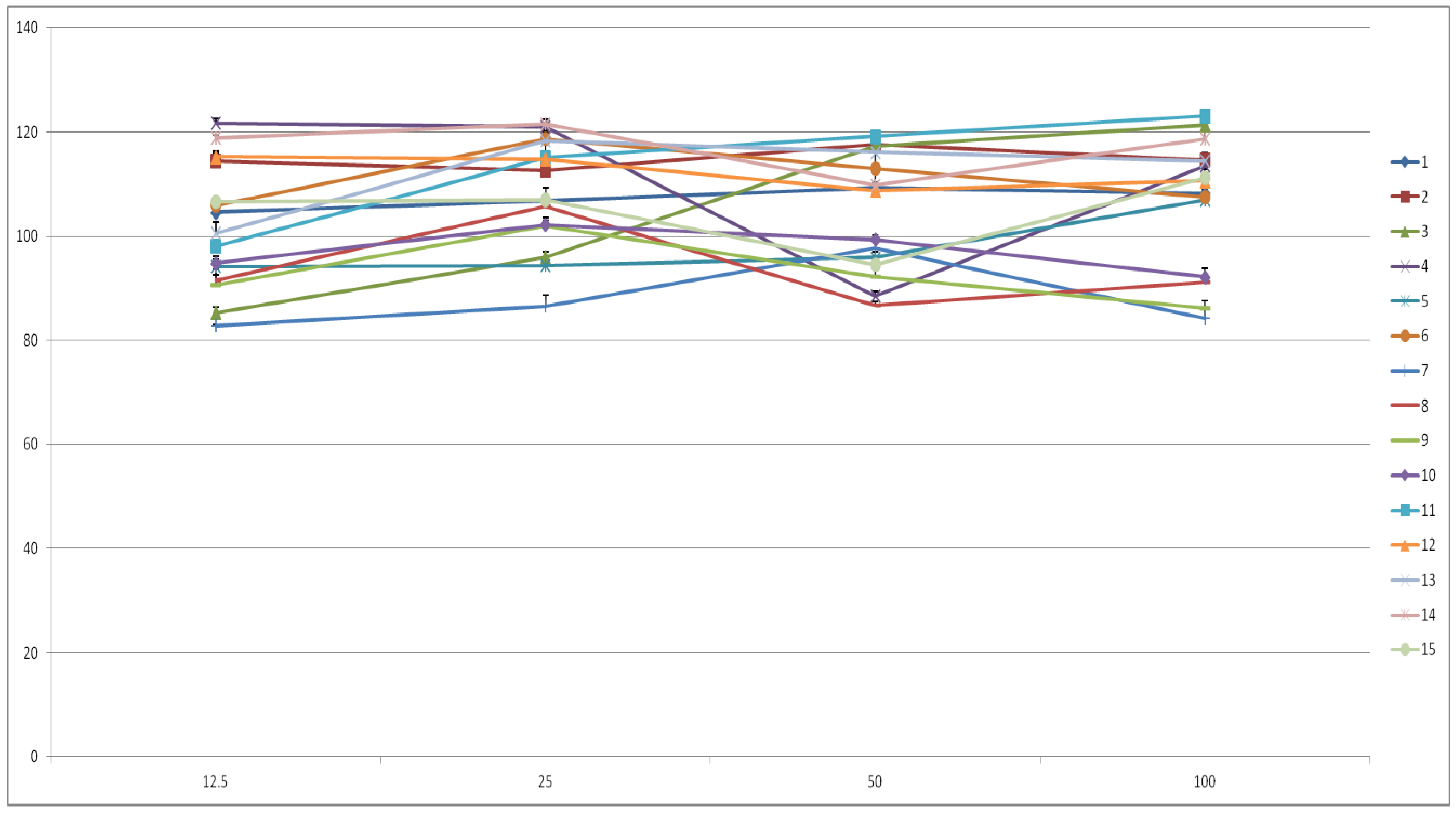
2.3. Cell Viability and Inhibition of Cancer Cell Proliferation
| Compounds | DPPH radical scavenging activity SC50 (μM/mL) | Superoxide scavenging activity SC50 (μM/mL) |
|---|---|---|
| 1 | 23.05 ± 0.92 d,e | 13.47 ± 0.05 b |
| 2 | 51.49 ± 0.77 g | 11.71 ± 0.36 b |
| 3 | 21.65 ± 0.72 d,e | 22.68 ± 0.35 d |
| 4 | 13.41 ± 0.10 c | 10.81 ± 0.07 c |
| 5 | >100 i | >100 e |
| 6 | 36.34 ± 0.93 f | 13.68 ± 0.74 b, c |
| 7 | 21.63 ± 0.70 e | 3.85 ± 0.03 a |
| 8 | 3.12 ± 0.05 a | 0.29 ± 0.69 a |
| 9 | 2.97 ± 0.04 a | 0.09 ± 0.02 a |
| 10 | 3.29 ± 0.26 a | 0.16 ± 0.02 a |
| 11 | 49.09 ± 0.92 g | 12.18 ± 0.14 b,c |
| 12 | 60.96 ± 0.70 h | 12.43 ± 0.24 b,c |
| 13 | 17.06 ± 0.15 d | 3.17 ± 0.78 a |
| 14 | 36.87 ± 0.44 f | 2.59 ± 0.75 a |
| 15 | 19.45 ± 0.05 d,e | 1.45 ± 0.24 a |
| Ascorbic acid | 8.22 ± 0.64 b | - |
| Allopurinol | - | 2.39±0.09 a |
| Compounds | Anti-proliferative activity of LNCaP cancer cell line IC50 (μM/mL) | Anti-proliferative activity of Du-145 cancer cell line IC50 (μM/mL) |
|---|---|---|
| 1 | >100 c | >100 a |
| 2 | >100 c | >100 a |
| 3 | >100 c | >100 a |
| 4 | >100 c | >100 a |
| 5 | >100 c | >100 a |
| 6 | >100 c | >100 a |
| 7 | >100 c | >100 a |
| 8 | 66.35 ± 1.44 b | >100 a |
| 9 | 43.08 ± 0.63 a | >100 a |
| 10 | 40.56 ± 1.41 a | >100 a |
| 11 | >100 c | >100 a |
| 12 | >100 c | >100 a |
| 13 | >100 c | >100 a |
| 14 | >100 c | >100 a |
| 15 | >100 c | >100 a |
3. Experimental
3.1. General Methods
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Antioxidant Activity
3.4.1. Measurement of DPPH Radical Scavenging Activity
3.4.2. Xanthine Oxidase Superoxide Scavenging Activity
3.5. Anti-Proliferation and Cytotoxicity Assays
3.5.1. Cell Culture
3.5.2. Measurement of Cell Viability
3.5.3. Anti-Proliferation Assays
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Zhang, Z.; Zhang, H.; Peter, K.E. Hamaelidaceae. In Flora of China; Science Press & Missouri Botanical Garden: Beijing, China and St. Louis, MO, USA, 2003; Volume 9, pp. 18–42. [Google Scholar]
- Huafu, W.; Gordon, J.P.; Keith, H. Determination of hamameli-tannin, catechins and gallic acid in witch hazel bark, twig and leaf by HPLC. J. Pharm. Biomed. Anal. 2003, 33, 539–544. [Google Scholar] [CrossRef]
- Korting, H.C.; Schäfer-Korting, M.; Klövekorn, W.; Klövekorn, G.; Martin, C.; Laux, P. Comparative efficacy of hamamelis distillate and hydrocortisone cream in atopic eczema. Eur. J. Clin. Pharmacol. 1995, 48, 461–465. [Google Scholar]
- Dauer, A.; Metzner, P.; Schimmer, O. Proanthocyanidins from the bark of Hamamelis virginiana exhibit antimutagenic properties against nitroaromatic compounds. Planta Med. 1998, 64, 324–327. [Google Scholar] [CrossRef]
- Dauer, A.; Rimpler, H.; Hensel, A. Polymeric proanthocyanidins from the bark of Hamamelis virginiana L. Planta Med. 2003, 69, 89–91. [Google Scholar] [CrossRef]
- Hideo, I.; Hideko, M.; Ideko, M.; Noriko, O.; Ryo, K. 3-O-feruloyl-4-O-caffeoylquinic acid from coffee beans. Phytochemistry 1985, 24, 630–632. [Google Scholar] [CrossRef]
- Akdemir, Z.S.; Tatli, I.I.; Saracoglu, I.; Ismailoglu, U.B.; Sahin-Erdemli, I.; Caliş, I. Polyphenolic compounds from Geranium prat-ense and their free radical scavenging activities. Phytochemistry 2001, 56, 189–193. [Google Scholar]
- Zamarrud, A.I.; Hussain, H.; Ahmad, V.U.; Qaiser, M.; Amyn, A.; Mohammad, F.V. Two new antioxidant Bergen-in derivatives from the stem of Rivea hypocrateriformis. Fitoterapia 2011, 82, 722–725. [Google Scholar] [CrossRef]
- Dong, W.; Zhu, H.T.; Zhang, Y.J.; Yang, C.R. A carbon-carbon-coupled dimeric bergenin derivative biotransformed by Pleu-rotus ostreatus. Bioorg. Med. Chem. Lett. 2005, 15, 4073–4075. [Google Scholar] [CrossRef]
- Lee, M.W.; Tanaka, T.; Nonaka, G.I.; Nishioka, I. Hirsunin, an ellagitannin with a diarylheptanoid moiety, from Alnus hirsuta var. Microphylla. Phytochemistry 1992, 31, 967–970. [Google Scholar]
- Jin, Z.X.; Ito, H.; Yoshida, T. Dimeric and trimeric ellagitannins from corylus heterophylla. Phytochemistry 1998, 48, 333–338. [Google Scholar]
- Jeng, D.S.; Toshihiko, O.; Shunro, K.; Mitsuo, N. Tannin antioxidants from Osbeckia chinensis. Phytochemistry 1988, 27, 1315–1319. [Google Scholar]
- Lommen, A.; Godejohann, M.; Venema, D.P.; Hollman, P.C.H.; Spraul, M. Application of directly coupled HPLC-NMR-MS to the identification and confirmation of quercetin glycosides and phloretin glycosides in apple peel. Anal. Chem. 2000, 72, 1793–1797. [Google Scholar]
- Philippe, O.D.B.; Dijoux, M.G.; Gilbert, C.; Anne-marie, M.I. Quercitrin T-sulphate fromleaves of leea G uinensis. Phytochemistry 1998, 47, 1171–1173. [Google Scholar]
- Mohamed, B.; Aziz, A.; Christian, R. Regio- and ster-eoselective synthesis of the major metabolite of quercetin, quercetin-3-O-β-d-glucuronide. Tetrahedron Lett. 2002, 43, 6263–6266. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, Y.H.; Moon, B.H.; Lee, E.J.; Hong, S.W.; Lim, Y.H. Substitution effect of hydroxyl groups on the 1H and 13C Chemical Shifts in Hydroxyflavonols. Bull. Korean Chem. Soc. 2008, 29, 1597–1600. [Google Scholar] [CrossRef]
- Lee, J.I.; Kong, C.S.; Jung, M.E.; Hong, J.W.; Noh, I.; Seo, Y.W. Peroxynitrite-scavenging activity of the halophyte limon-ium tetragonum. Ocean Polar Res. 2011, 33, 185–191. [Google Scholar]
- Park, J.H.; Li, C.; Hu, W.; Wang, M.H. Antioxidant and Free Radical Scavenging Activity of Different Fractions from Hawthorn Fruit. J. Food Sci. Nutr. 2010, 5, 44–50. [Google Scholar] [CrossRef]
- Hobbs, A.; Higgs, A.; Moncada, S. Inhibition of nitric oxide synthase as a potential therapeutic target. Ann. Rev. Pharm. Toxicol. 1999, 39, 191–220. [Google Scholar] [CrossRef]
- Alessandra, R.; Venera, C.; Laura, L.; Luca, V.; Angelo, V.; Juan, A.G. Antioxidant activity and antiproliferative action of methanolic extract of Geum quellyon Sweet roots in human tumor cell lines. J. Ethnopharmacol. 2005, 100, 323–332. [Google Scholar] [CrossRef]
- Hatano, T.; Edamatsu, R.; Hiramatsu, M.; Mori, A.; Fujita, Y.; Yasuhara, T. Effects of the interaction of tannins with co-exist substances. IV. Effects of tannins and ralated polyphenols on superoxide anion radical, and on 1,1-diphenyl-2-picrylhydrazyl radical. Chem. Pharm. Bull. 1989, 37, 2016–2021. [Google Scholar]
- Christin, A.; Martine, T.; Jacques, H. Superoxide anion involvement in NBT reduction by NADPH-cytochrome P-450 reductase. FEBS Lett. 1978, 89, 26–28. [Google Scholar] [CrossRef]
- Toshiyuki, F.; Hideyuki, I.; Takashi, Y. Antioxidative polyphen-ols from walnuts (Juglans regia L.). Phytochemistry 2003, 63, 795–801. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for the cellular growth and survival. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Yi, Z.C.; Liu, Y.Z.; Li, H.X.; Yin, Y.; Zhuang, F.Y.; Fan, Y.B.; Wang, Z. Tellimagrandin I enhances gap junctional communication and attenuates the tumor phenotype of human cervical carcinoma HeLa cells in vitro. Cancer Lett. 2006, 242, 77–87. [Google Scholar] [CrossRef]
- Miyamoto, K.; Nomura, M.; Murayama, T.; Furukawa, T.; Hatano, T.; Yoshida, T.; Koshiura, R.; Okuda, T. Antitumor activities of ellagitannins against sarcoma-180 in mice. Biol. Pharm. Bull. 1993, 16, 379–387. [Google Scholar] [CrossRef]
- Ken, S.F. Recent progress in ellagitannin chemistry. Phytochemistry 2005, 66, 1984–2000. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–15 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, M.H.; Ha, S.Y.; Oh, M.H.; Kim, H.H.; Kim, S.R.; Lee, M.W. Anti-Oxidative and Anti-Proliferative Activity on Human Prostate Cancer Cells Lines of the Phenolic Compounds from Corylopsis coreana Uyeki. Molecules 2013, 18, 4876-4886. https://doi.org/10.3390/molecules18054876
Kim MH, Ha SY, Oh MH, Kim HH, Kim SR, Lee MW. Anti-Oxidative and Anti-Proliferative Activity on Human Prostate Cancer Cells Lines of the Phenolic Compounds from Corylopsis coreana Uyeki. Molecules. 2013; 18(5):4876-4886. https://doi.org/10.3390/molecules18054876
Chicago/Turabian StyleKim, Manh Heun, Sung Yi Ha, Myeong Hwan Oh, Han Hyuk Kim, So Ra Kim, and Min Won Lee. 2013. "Anti-Oxidative and Anti-Proliferative Activity on Human Prostate Cancer Cells Lines of the Phenolic Compounds from Corylopsis coreana Uyeki" Molecules 18, no. 5: 4876-4886. https://doi.org/10.3390/molecules18054876




