2'-Deoxythymidine Adducts from the Anti-HIV Drug Nevirapine
Abstract
:1. Introduction
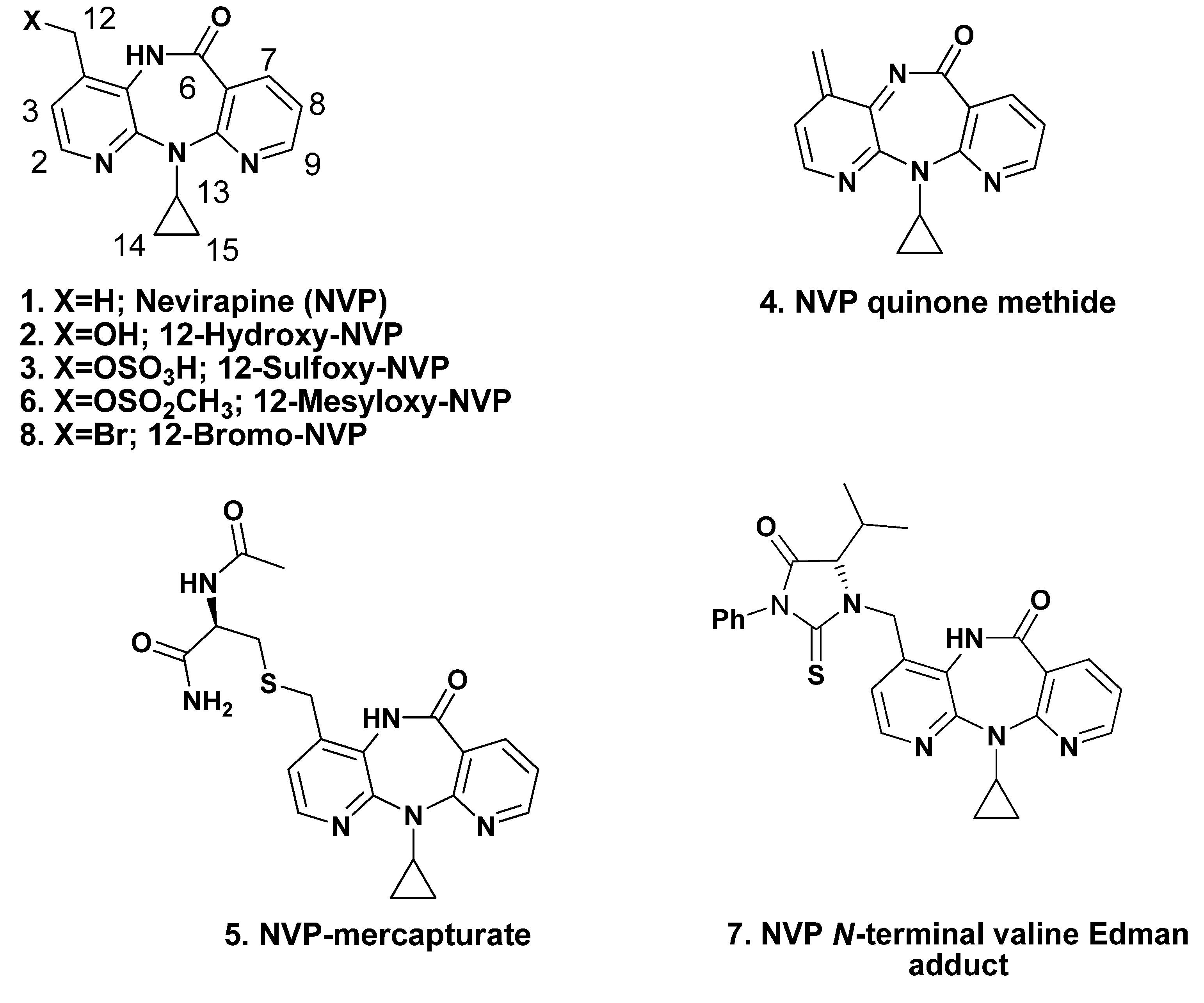
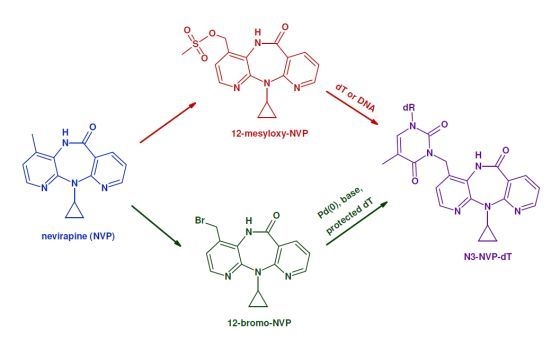
2. Results and Discussion
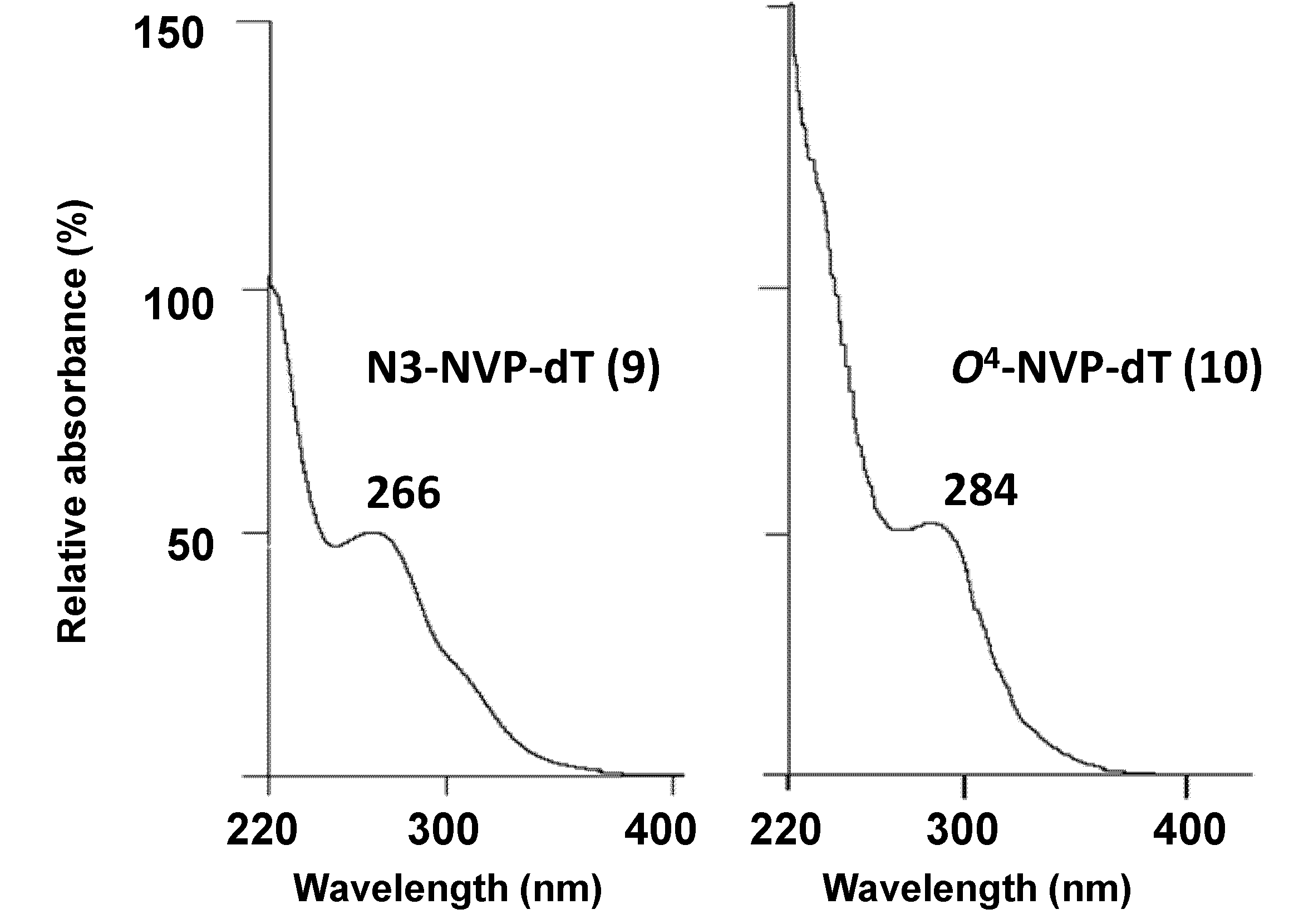
2.1. Synthesis and Structural Characterization of NVP-dT Adduct Standards
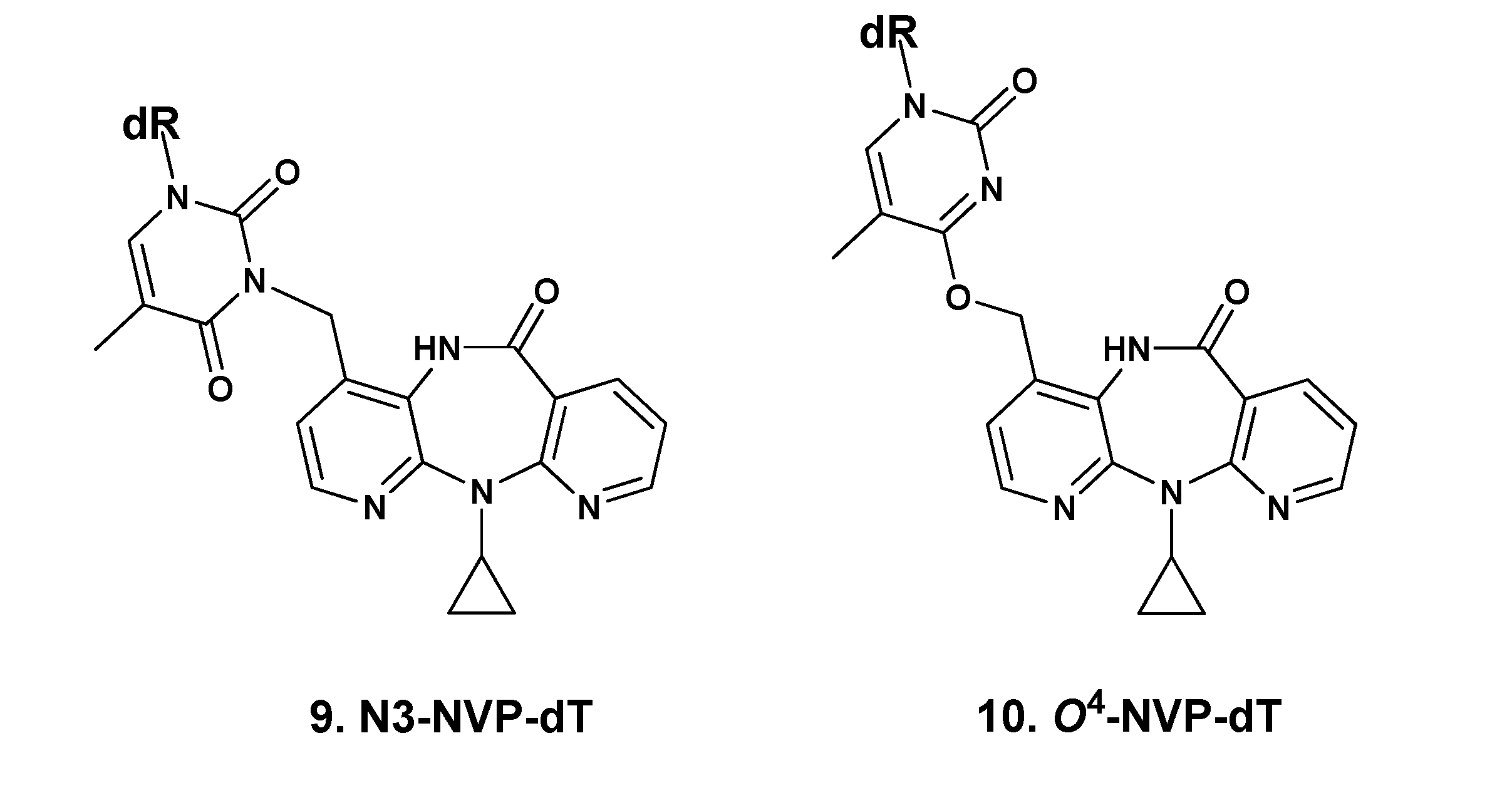

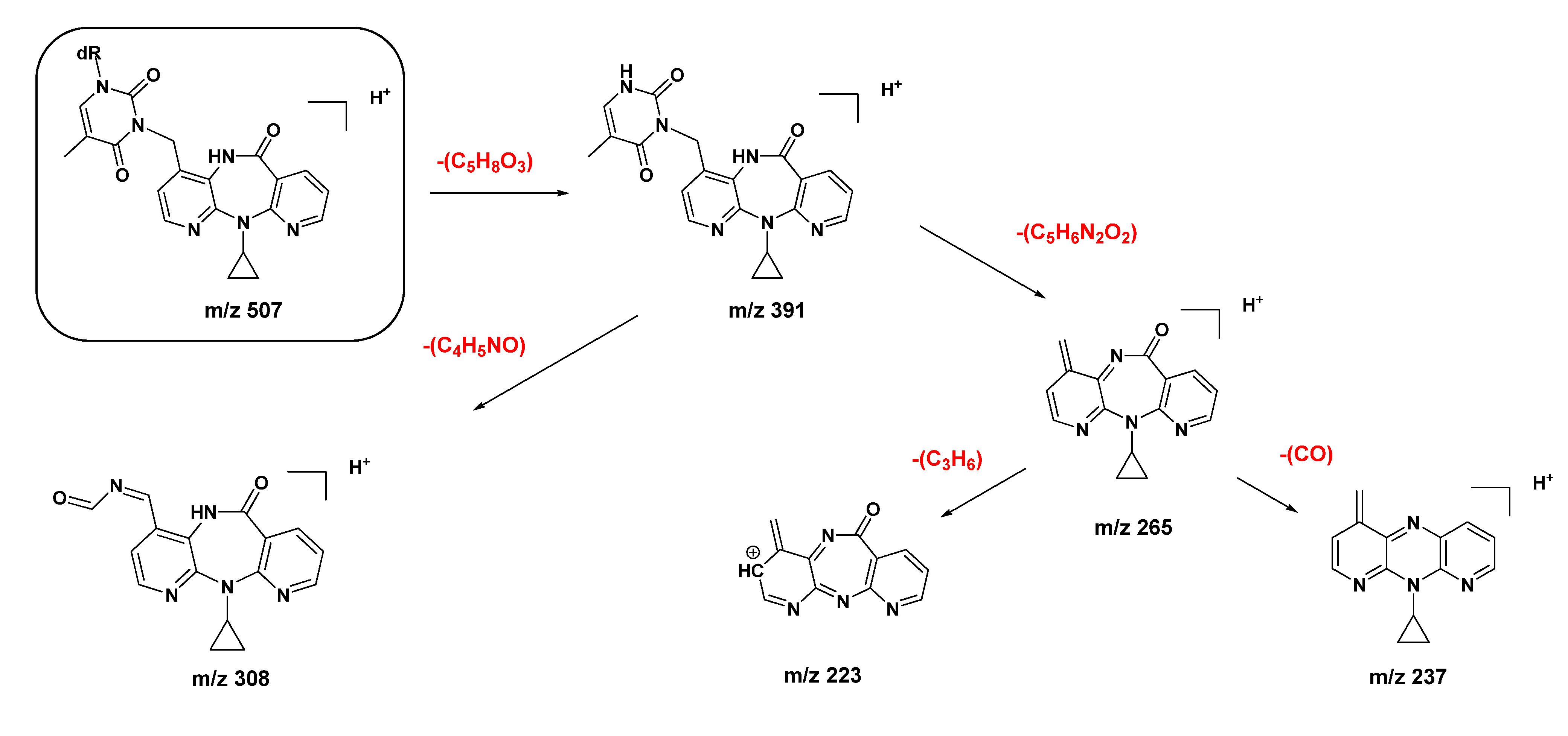
| N3-methyl-dT [b] | O2-methyl-dT [c] | O4-methyl-dT [d] | N3-NVP-dT (9) | O4-NVP-dT (10) | |
|---|---|---|---|---|---|
| UV(λmax, nm) | 267, 235 [e] | 259, 237 (pH 1) | 280, 241 [f] | 266 [g] | 284 [g] |
| 257, 239 | |||||
| (pH 13) | |||||
| δ (ppm) | |||||
| H1' | 6.27 | 6.08 | 6.24 | 6.17 | 6.66 |
| H2' | 2.14 | 2.12–2.17 | 2.0–2.19 | 2.50 | NA [h] |
| H3' | 3.65–4.76 | 4.19–4.27 | 3.84 | 3.77–3.79 | NA |
| H4' | 3.65–4.76 | 3.70–3.80 | 4.22 | 4.26 | 5.33 |
| H5' | 3.55–3.60 | 3.47–3.66 | 3.59–3.81 | 3.50 | NA |
| H6 | 7.83 | 7.80 | 8.01 | 7.90 | 8.55 |
| dT-CH3 | 1.87 | 1.78 | 1.88 | 1.90 | NA |
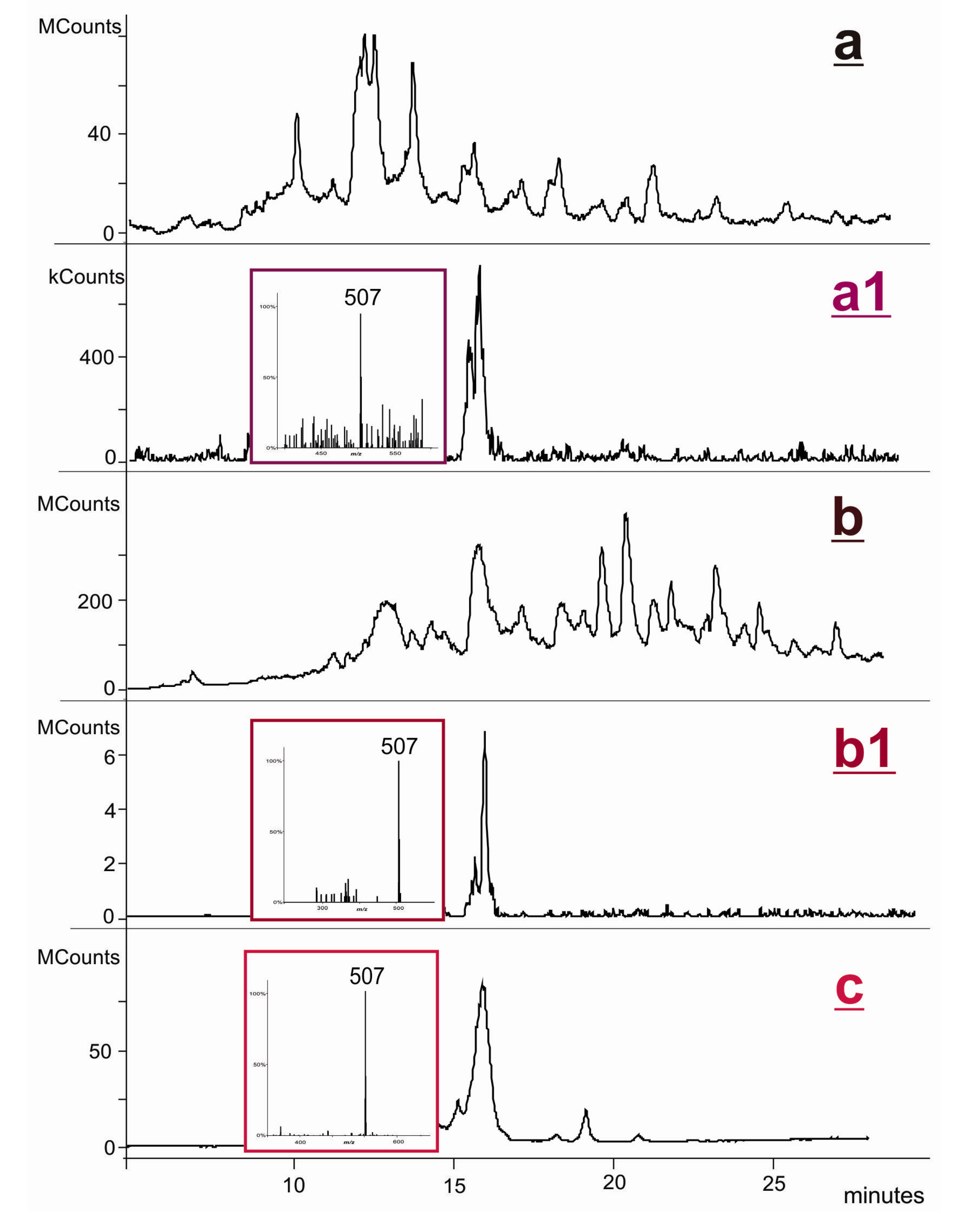
2.2. Covalent Modification of dT and DNA with 12-Mesyloxy-NVP (6)

3. Experimental
3.1. Chemicals
3.2. Instrumentation
3.2.1. Analytical and Semipreparative HPLC
3.2.2. NMR
3.2.3. LC-ESI-MS
3.3. Syntheses
3.3.1. Palladium-Mediated Coupling of 12-Bromo-NVP (8) with 3',5'-O-bis(tert-butyldimethylsilyl)-2'-deoxythymidine
3.3.2. Reaction of 2'-Deoxythymidine with 12-Mesyloxy-NVP (6)
3.3.3. Reaction of 12-Mesyloxy-NVP (6) with DNA
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Guidelines on HIV/AIDS. Available online: http://www.who.int/rpc/guidelines/hiv_aids/en/index.html (accessed on 10 March 2013).
- Powles, T.; Robinson, D.; Stebbing, J.; Shamash, J.; Nelson, M.; Gazzard, B.; Mandelia, S.; Møller, H.; Bower, M. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J. Clin. Oncol. 2009, 27, 884–890. [Google Scholar] [CrossRef]
- Marseille, E.; Kahn, J.G.; Mmiro, F.; Guay, L.; Musoke, P.; Fowler, M.G.; Jackson, J.B. Cost effectiveness of single-dose nevirapine regimen for mothers and babies to decrease vertical HIV-1 transmission in sub-Saharan Africa. Lancet 1999, 354, 803–809. [Google Scholar]
- Jackson, J.B.; Musoke, P.; Fleming, T.; Guay, L.A.; Bagenda, D.; Allen, M.; Nakabiito, C.; Sherman, J.; Bakaki, P.; Owor, M.; et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet 2003, 362, 859–868. [Google Scholar]
- Lallemant, M.; Jourdain, G.; le Coeur, S.; Mary, J.Y.; Ngo-Giang-Huong, N.; Koetsawang, S.; Kanshana, S.; McIntosh, K.; Thaineua, V.; Perinatal HIV Prevention Trial (Thailand) Investigators. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N. Engl. J. Med. 2004, 351, 217–228. [Google Scholar] [CrossRef]
- Lockman, S.; Shapiro, R.L.; Smeaton, L.M.; Wester, C.; Thior, I.; Stevens, L.; Chand, F.; Makhema, J.; Moffat, C.; Asmelash, A.; et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N. Engl. J. Med. 2007, 356, 135–147. [Google Scholar] [CrossRef]
- Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. Available online: http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf (accessed on 10 March 2013).
- Ruiz, L.; Negredo, E.; Domingo, P.; Paredes, R.; Francia, E.; Balagué, M.; Gel, S.; Bonjoch, A.; Fumaz, C.R.; Johnston, S.; et al. Antiretroviral treatment simplification with nevirapine in protease inhibitor-experienced patients with HIV-associated lipodystrophy: 1-year prospective follow-up of a multicenter, randomized, controlled study. J. Acquir. Immune Defic. Syndr. 2001, 27, 229–236. [Google Scholar]
- Clotet, B.; van der Valk, M.; Negredo, E.; Reiss, P. Impact of nevirapine on lipid metabolism. J. Acquir. Immune. Defic. Syndr. 2003, 34 (Suppl. 1), S79–S84. [Google Scholar]
- Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. Available online: http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf (accessed on 10 March 2013).
- FDA. Approval of Viramune XR (nevirapine) 400 mg extended release tablet. 2011. Available online: http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm248800.htm (accessed on 10 March 2013).
- Pollard, R.B.; Robinson, P.; Dransfield, K. Safety profile of nevirapine, a nonnucleoside reverse transcriptase inhibitor for the treatment of human immunodeficiency virus infection. Clin. Ther. 1998, 20, 1071–1092. [Google Scholar]
- Murphy, R.L. Defining the toxicity profile of nevirapine and other antiretroviral drugs. J. Acquir. Immune Defic. Syndr. 2003, 34 (Suppl. 1), S15–S20. [Google Scholar] [CrossRef]
- Mirochnick, M.; Clarke, D.F.; Dorenbaum, A. Nevirapine: pharmacokinetic considerations in children and pregnant women. Clin. Pharmacokinet. 2000, 39, 281–293. [Google Scholar] [CrossRef]
- Waters, L.; John, L.; Nelson, M. Non-nucleoside reverse transcriptase inhibitors: A review. Int. J. Clin. Pract. 2007, 61, 105–118. [Google Scholar] [CrossRef]
- Ton, Q.; Frenkel, L. HIV drug resistance in mothers and infants following use of antiretrovirals to prevent mother-to-child transmission. Curr. HIV Res. 2013, 11, 126–136. [Google Scholar] [CrossRef]
- PDR staff, VIRAMUNE® (nevirapine). In Physicians’ Desk Reference, 63rd ed; Physicians’ Desk Reference Inc: Montvale, NJ, USA, 2009; pp. 873–881.
- Popovic, M.; Caswell, J.L.; Mannargudi, B.; Shenton, J.M.; Uetrecht, J.P. Study of the sequence of events involved in nevirapine-induced skin rash in Brown Norway rats. Chem. Res. Toxicol. 2006, 19, 1205–1214. [Google Scholar]
- Wen, B.; Chen, Y.; Fitch, W.L. Metabolic activation of nevirapine in human liver microsomes: Dehydrogenation and inactivation of cytochrome P450 3A4. Drug Metab. Dispos. 2009, 37, 1557–1562. [Google Scholar]
- Chen, J.; Mannargudi, B.M.; Xu, L.; Uetrecht, J. Demonstration of the metabolic pathway responsible for nevirapine-induced skin rash. Chem. Res. Toxicol. 2008, 21, 1862–1870. [Google Scholar]
- Srivastava, A.; Lian, L.-Y.; Maggs, J.L.; Chaponda, M.; Pirmohamed, M.; Williams, D.P.; Park, B.K. Quantifying the metabolic activation of nevirapine in patients by integrated applications of NMR and mass spectrometries. Drug Metab. Dispos. 2010, 38, 122–132. [Google Scholar]
- Sharma, A.M.; Li, Y.; Novalen, M.; Hayes, M.A.; Uetrecht, J. Bioactivation of nevirapine to a reactive quinone methide: Implications for liver injury. Chem. Res. Toxicol. 2012, 25, 1708–1719. [Google Scholar]
- Sharma, A.M.; Klarskov, K.; Uetrecht, J. Nevirapine bioactivation and covalent binding in the skin. Chem. Res. Toxicol. 2013, 26, 410–421. [Google Scholar]
- Glatt, H. Sulfation and sulfotransferases 4. Bioactivation of mutagens via sulfation. FASEB J. 1997, 11, 314–321. [Google Scholar]
- Antunes, A.M.M.; Duarte, M.P.; Santos, P.P.; Gamboa da Costa, G.; Heinze, T.M.; Beland, F.A.; Marques, M.M. Synthesis and characterization of DNA adducts from the HIV reverse transcriptase inhibitor nevirapine. Chem. Res. Toxicol. 2008, 21, 1443–1456. [Google Scholar]
- Antunes, A.M.M.; Godinho, A.L.A.; Martins, I.L.; Justino, G.C.; Beland, F.A.; Marques, M.M. Amino acid adduct formation by the nevirapine metabolite, 12-hydroxynevirapine—A possible factor in nevirapine toxicity. Chem. Res. Toxicol. 2010, 23, 888–899. [Google Scholar]
- Antunes, A.M.M.; Godinho, A.L.A.; Martins, I.L.; Oliveira, M.C.; Gomes, R.A.; Coelho, A.V.; Beland, F.A.; Marques, M.M. Protein adducts as prospective biomarkers of nevirapine toxicity. Chem. Res. Toxicol. 2010, 23, 1714–1725. [Google Scholar]
- Caixas, U.; Antunes, A.M.M.; Marinho, A.T.; Godinho, A.L.A.; Grilo, N.M.; Marques, M.M.; Oliveira, M.C.; Branco, T.; Monteiro, E, C.; Pereira, S.A. Evidence for nevirapine bioactivation in man: Searching for the first step in the mechanism of nevirapine toxicity. Toxicology 2012, 301, 33–39. [Google Scholar]
- Phillips, D.H. DNA adducts as markers of exposure and risk. Mutat. Res. 2005, 577, 284–292. [Google Scholar]
- Farmer, P.B.; Brown, K.; Tompkins, E.; Emms, V.L.; Jones, D.J.L.; Singh, R.; Phillips, D.H. DNA adducts: Mass spectrometry methods and future prospects. Toxicol. Appl. Pharmacol. 2005, 207, S293–S301. [Google Scholar]
- Fang, Q.; Kanugula, S.; Tubbs, J.L.; Tainer, J.A.; Pegg, A.E. Repair of O4-alkylthymine by O6-alkylguanine-DNA alkyltransferases. J. Biol. Chem. 2010, 285, 8185–8195. [Google Scholar]
- Lao, Y.; Yu, N.; Kassie, F.; Villalta, P.W.; Hecht, S.S. Formation and accumulation of pyridyloxobutyl DNA adducts in F344 rats chronically treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of its metabolite, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem. Res. Toxicol. 2007, 20, 235–245. [Google Scholar]
- Den Engelse, L.; de Graaf, A.; de Brij, R.-J.; Menkveld, G.J. O2- and O4-ethylthymine and the ethylphosphotriester dTp(Et)dT are highly persistent DNA modifications in slowly dividing tissues of the ethylnitrosourea-treated rat. Carcinogenesis 1987, 8, 751–757. [Google Scholar]
- Bhanot, O.S.; Solomon, J.J. The role of mutagenic metal ions in mediating in vitro mispairing by alkylpyrimidines. Environ. Health Perspect. 1994, 102 (Suppl. 3), 81–90. [Google Scholar]
- Grevatt, P.C.; Donahue, J.M.; Bhanot, O.S. The role of N3-ethyldeoxythymidine in mutagenesis and cytotoxicity by ethylating agents. J. Biol. Chem. 1991, 266, 1269–1275. [Google Scholar]
- Lakshman, M.K. Synthesis of biologically important nucleoside analogs by palladium-catalyzed C-N bond-formation. Curr. Org. Synth. 2005, 2, 83–112. [Google Scholar]
- Kimura, T.; Watanabe, K.; Tateoka, Y.; Kondo, S.; Ho, I.K.; Yamamoto, I. Preparation and pharmacological evaluation of N3-substituted thymidine derivatives as central depressants. Chem. Pharm. Bull. 1993, 41, 1180–1182. [Google Scholar]
- Huang, J.J.; Ragouzeos, A.; Rideout, J.L. A novel synthesis of 3′-deoxy-3′-nitrothymidine via nucleophilic substitution with nitrite anion. J. Heterocycl. Chem. 1995, 32, 691–695. [Google Scholar]
- Miah, A.; Reese, C.B.; Song, Q. Convenient intermediates for the preparation of C-4 modified derivatives of pyrimidine nucleosides. Nucleosides Nucleotides 1997, 16, 53–65. [Google Scholar]
- Chang, C.-J.; DaSilva Gomes, J.; Byrn, S.R. Chemical modification of deoxyribonucleic acids: A direct study by carbon-13 nuclear magnetic resonance spectroscopy. J. Org. Chem. 1983, 48, 5151–5160. [Google Scholar]
- Lawley, P.D.; Orr, D.J.; Shah, S.A.; Farmer, P.B.; Jarman, M. Reaction products from N-methyl-N-nitrosourea and deoxyribonucleic acid containing thymidine residues. Synthesis and identification of a new methylation product, O4-methylthymidine. Biochem. J. 1973, 135, 193–201. [Google Scholar]
- Bhanot, O.S.; Grevatt, P.C.; Donahue, J.M.; Gabrielides, C.N.; Solomon, J.J. Incorporation of dA opposite N3-ethylthymidine terminates in vitro DNA synthesis. Biochemistry 1990, 29, 10357–10364. [Google Scholar]
- Perrin, D.D.; Armarego, W.L.F. Purification of Laboratory Chemicals, 3rd ed; Pergamon Press: Oxford, UK, 1988; pp. 1–391. [Google Scholar]
- Ogilvie, K.K.; Beaucage, S.L.; Schifman, A.L.; Theriault, N.Y.; Sadana, K.L. The synthesis of oligoribonucleotides. II. The use of silyl protecting groups in nucleoside and nucleotide chemistry. VII. Can. J. Chem. 1978, 56, 2768–2780. [Google Scholar]
- Heflich, R.H.; Morris, S.M.; Beranek, D.T.; McGarrity, L.J.; Chen, J.J.; Beland, F.A. Relationships between the DNA adducts and the mutations and sister-chromatid exchanges produced in Chinese hamster ovary cells by N-hydroxy-2-aminofluorene, N-hydroxy-N'-acetylbenzidine and 1-nitrosopyrene. Mutagenesis 1986, 1, 201–206. [Google Scholar]
- Sample Availability: Samples of the compounds 9 and 10 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Antunes, A.M.M.; Wolf, B.; Oliveira, M.C.; Beland, F.A.; Marques, M.M. 2'-Deoxythymidine Adducts from the Anti-HIV Drug Nevirapine. Molecules 2013, 18, 4955-4971. https://doi.org/10.3390/molecules18054955
Antunes AMM, Wolf B, Oliveira MC, Beland FA, Marques MM. 2'-Deoxythymidine Adducts from the Anti-HIV Drug Nevirapine. Molecules. 2013; 18(5):4955-4971. https://doi.org/10.3390/molecules18054955
Chicago/Turabian StyleAntunes, Alexandra M. M., Benjamin Wolf, M. Conceição Oliveira, Frederick A. Beland, and M. Matilde Marques. 2013. "2'-Deoxythymidine Adducts from the Anti-HIV Drug Nevirapine" Molecules 18, no. 5: 4955-4971. https://doi.org/10.3390/molecules18054955