Synthesis, Characterization and Activity Evaluation of Matrinic Acid Derivatives as Potential Antiproliferative Agents
Abstract
:1. Introduction
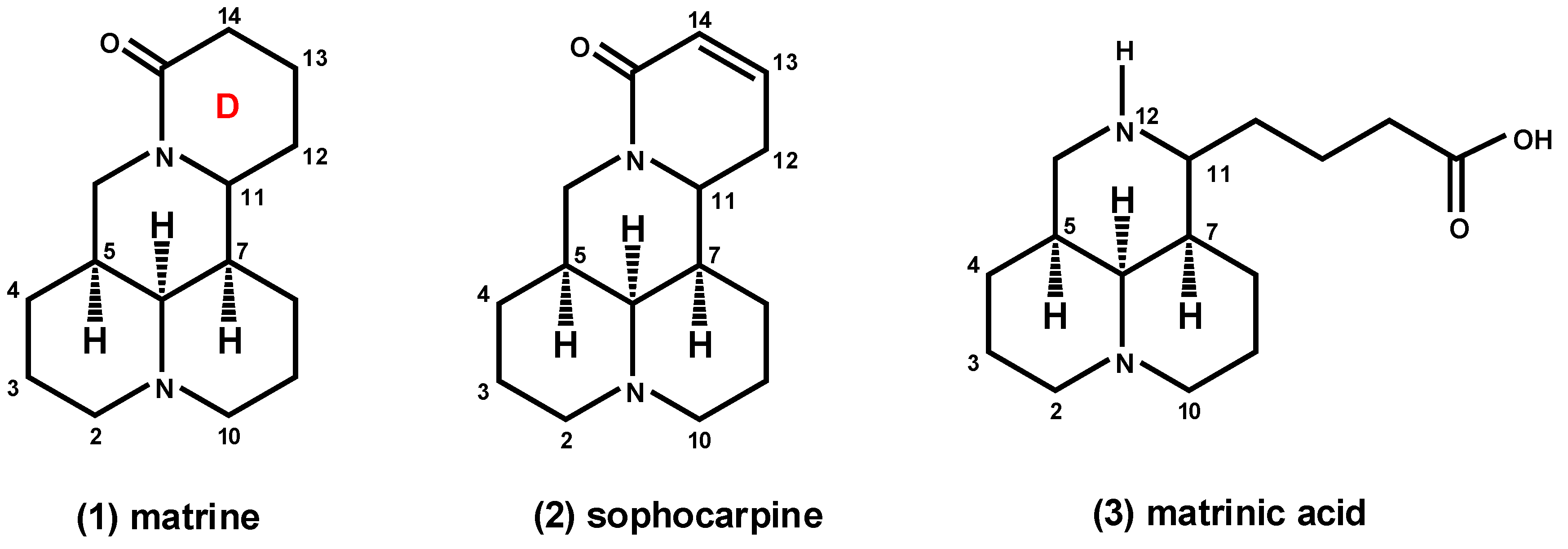
2. Results and Discussion
2.1. Chemistry
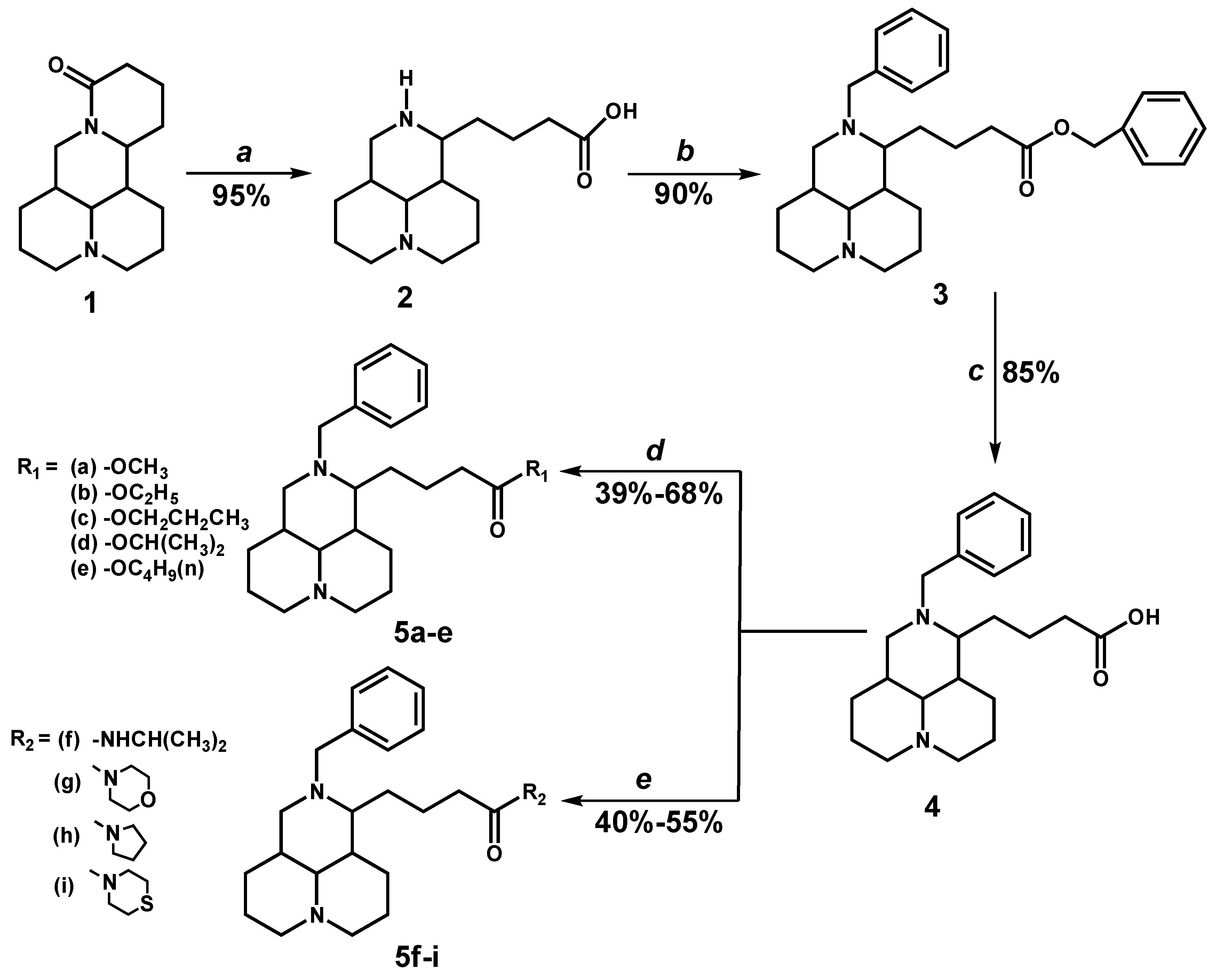
2.2. Antiproliferative Activity
 ), synthesized compounds (
), synthesized compounds (  ). Results are expressed as the mean ± SD of data obtained from three independent experiments.
). Results are expressed as the mean ± SD of data obtained from three independent experiments.
 ), synthesized compounds (
), synthesized compounds (  ). Results are expressed as the mean ± SD of data obtained from three independent experiments.
). Results are expressed as the mean ± SD of data obtained from three independent experiments.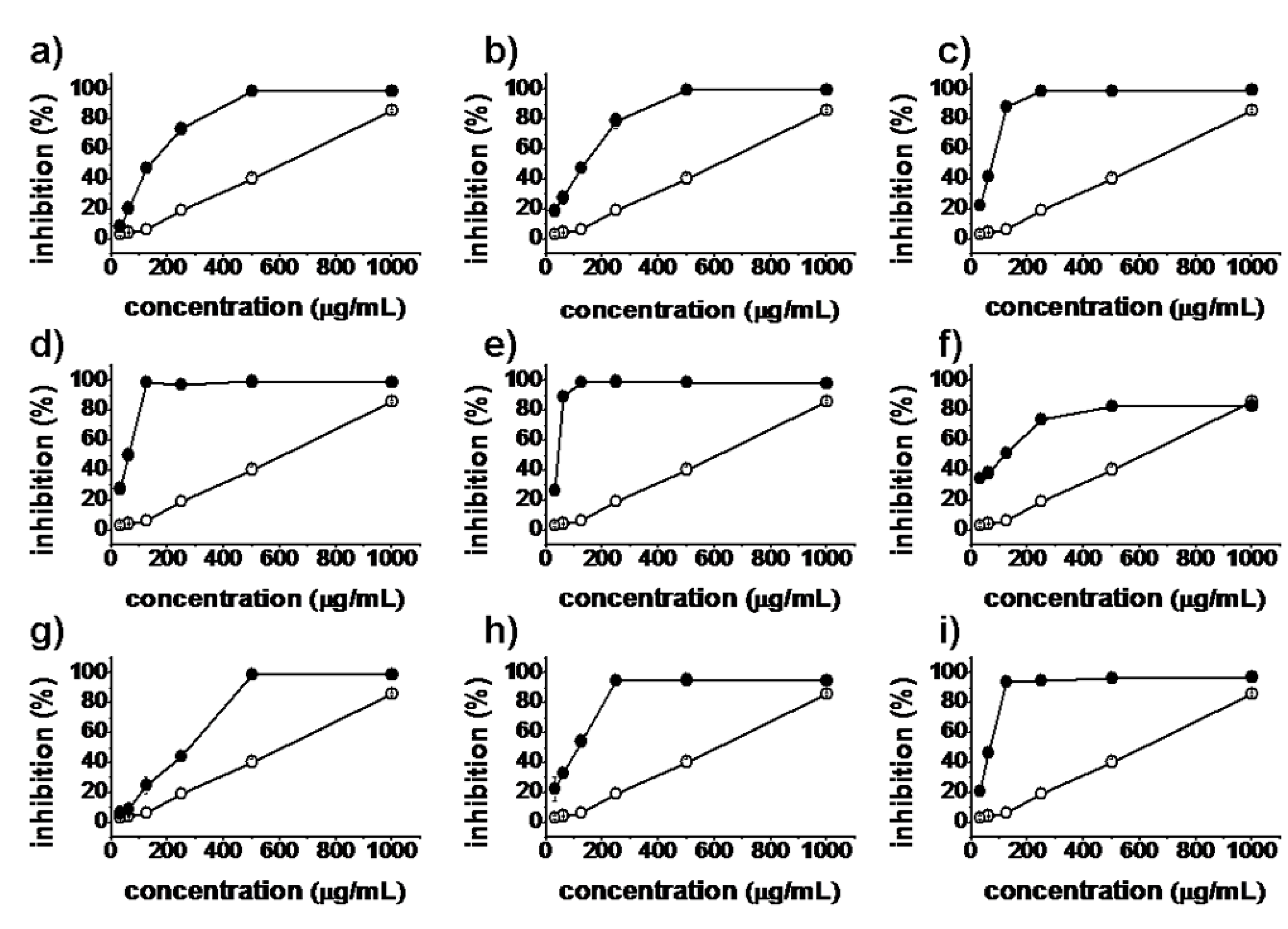
 ), synthesized compounds (
), synthesized compounds (  ). Results are expressed as the mean ± SD of data obtained from three independent experiments.
). Results are expressed as the mean ± SD of data obtained from three independent experiments.
 ), synthesized compounds (
), synthesized compounds (  ). Results are expressed as the mean ± SD of data obtained from three independent experiments.
). Results are expressed as the mean ± SD of data obtained from three independent experiments.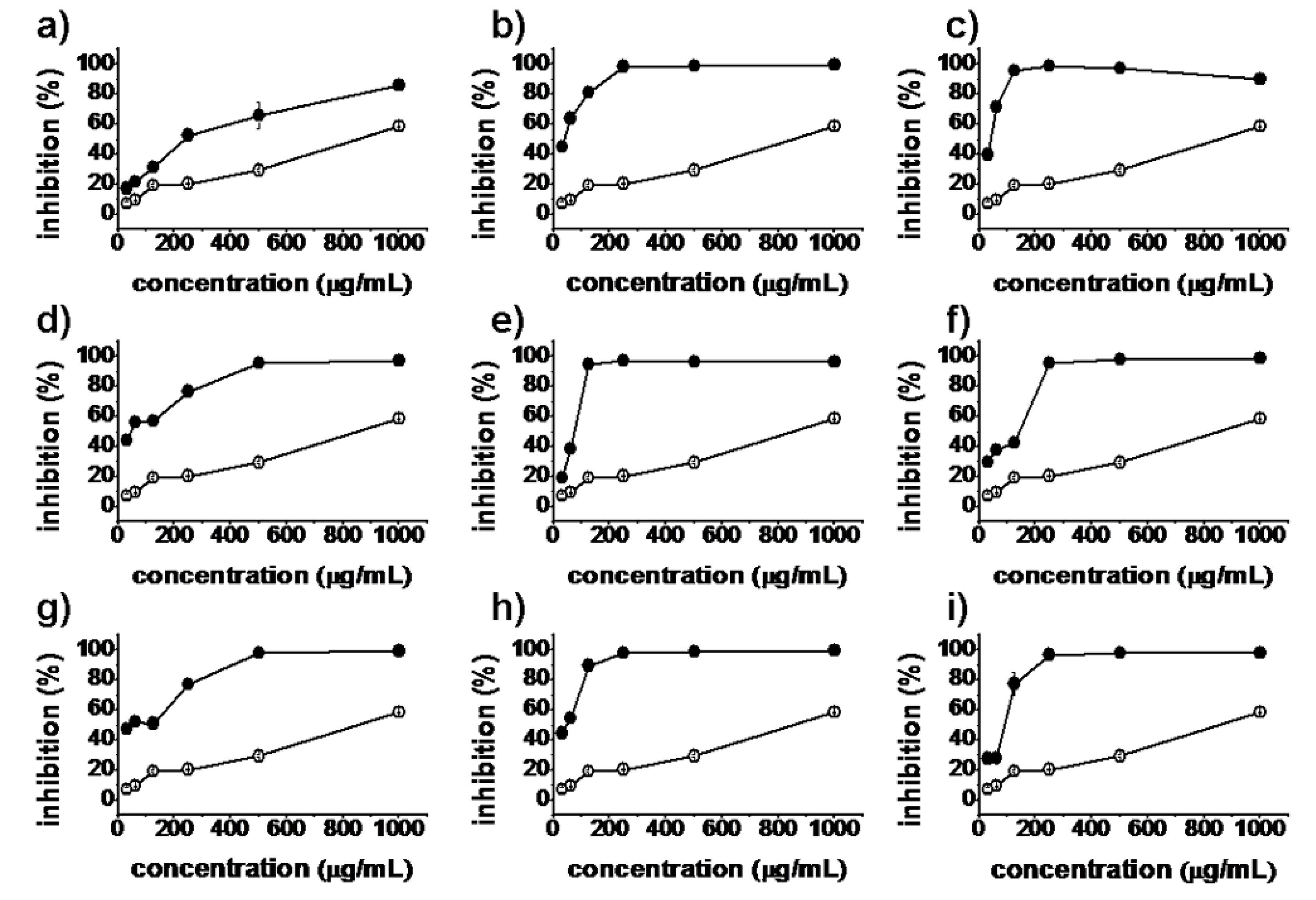
 ), synthesized compounds (
), synthesized compounds (  ). Results are expressed as the mean ± SD of data obtained from three independent experiments.
). Results are expressed as the mean ± SD of data obtained from three independent experiments.
 ), synthesized compounds (
), synthesized compounds (  ). Results are expressed as the mean ± SD of data obtained from three independent experiments.
). Results are expressed as the mean ± SD of data obtained from three independent experiments.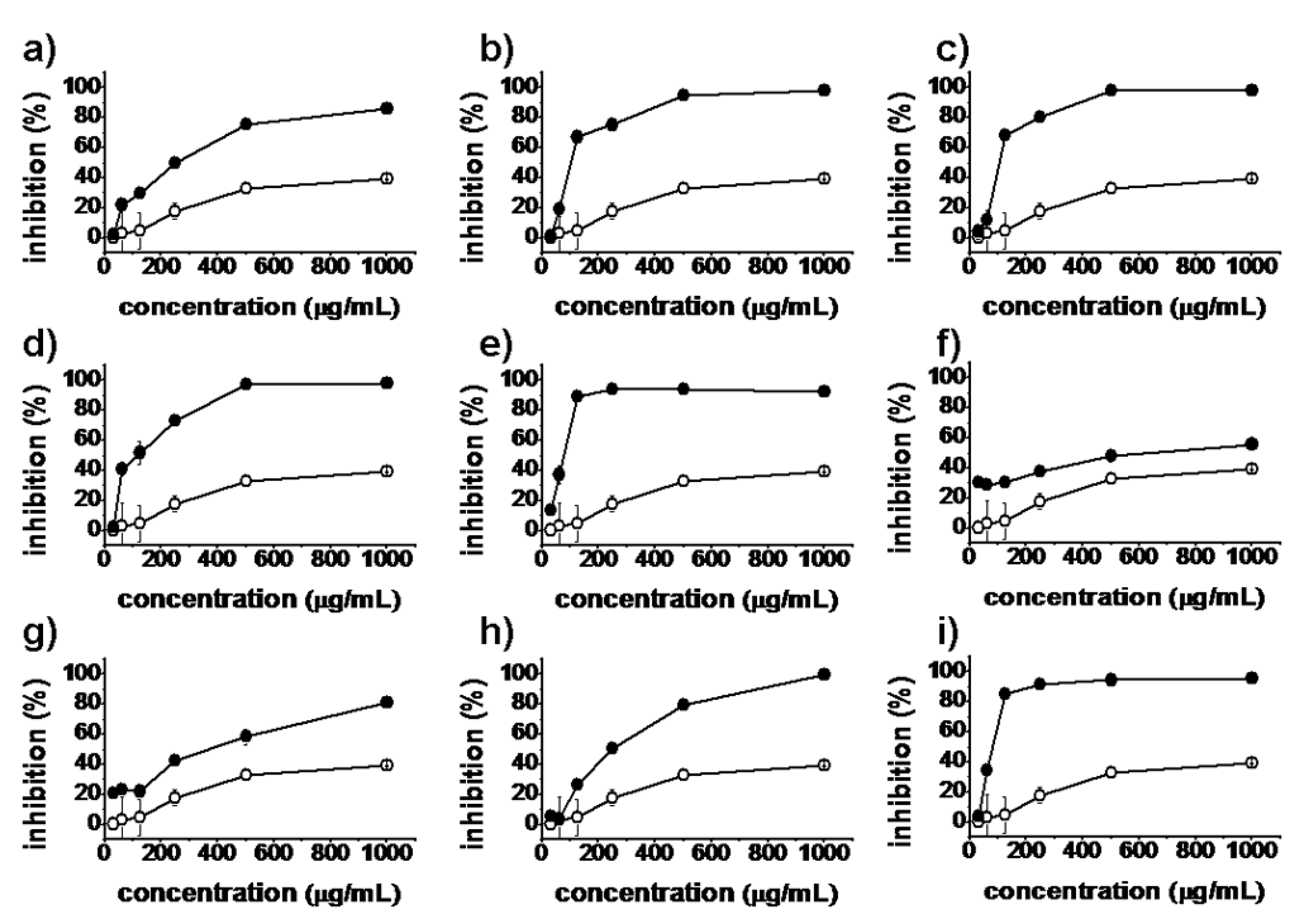
 ), synthesized compounds (
), synthesized compounds (  ). Results are expressed as the mean ± SD of data obtained from three independent experiments.
). Results are expressed as the mean ± SD of data obtained from three independent experiments.
 ), synthesized compounds (
), synthesized compounds (  ). Results are expressed as the mean ± SD of data obtained from three independent experiments.
). Results are expressed as the mean ± SD of data obtained from three independent experiments.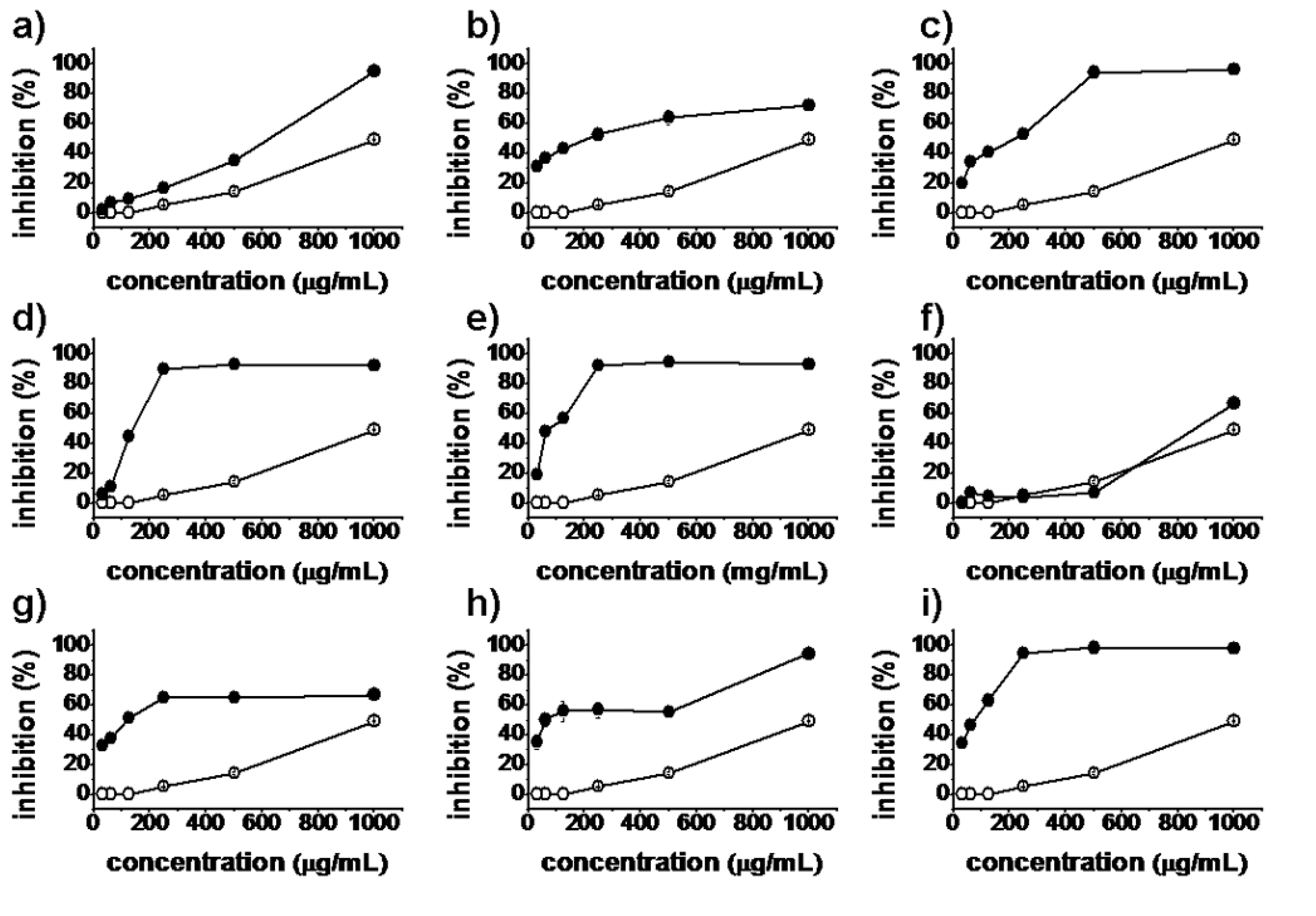
| Compound | ClogP a | IC50 (μg/mL) | |||
|---|---|---|---|---|---|
| A375 | A549 | HeLa | HepG2 | ||
| Matrine | 1.5 | 551.1 ± 22 | 1022.0 ± 16.5 | 1238.0 ± 5.7 | 1156.5 ± 11.9 |
| 2 | −0.704 | 1345.7 ± 125.6 | NA b | NA b | NA b |
| 4 | 1.572 | 853.1 ± 4.2 | NA b | 2201.5 ± 3.5 | 734.9 ± 24.4 |
| 5a | 4.218 | 138.7 ± 12.8 | 235.5 ± 1.6 | 223.5 ± 4.9 | 447.9 ± 34.4 |
| 5b | 4.747 | 116.7 ± 17 | 37.9 ± 2.3 | 121.2 ± 4.8 | 176.3 ± 5.6 |
| 5c | 5.276 | 60.7 ± 1.2 | 36.2 ± 1.3 | 118.8 ± 9.3 | 128.4 ± 2.3 |
| 5d | 5.056 | 58.5 ± 0.1 | 45.9 ± 0.1 | 102.9 ± 1.4 | 138.9 ± 9.2 |
| 5e | 5.805 | 37.0 ± 0.2 | 64.3 ± 6.2 | 75.5 ± 5.0 | 78.9 ± 0.4 |
| 5f | 3.796 | 82.8 ± 17.2 | 80.8 ± 2.7 | 758.2 ± 16.4 | 1383.3 ± 72.2 |
| 5g | 3.233 | 252 ± 1.4 | 47.0 ± 8.2 | 297.0 ± 27.6 | 143.6 ± 8.2 |
| 5h | 3.458 | 70.8 ± 7.0 | 41.5 ± 1.5 | 223.5 ± 4.9 | 100.9 ± 6.9 |
| 5i | 3.958 | 59.3 ± 0.9 | 70.6 ± 8.6 | 89.2 ± 0.6 | 61.0 ± 1.4 |
| Taxol | — | 31.3 ± 1.0 | 39.4 ± 0.9 | 18.3 ± 3.1 | 85.1 ± 6.2 |
3. Experimental
3.1. General
3.2. General Procedure for the Preparation of Matrinic Acid 2
3.3. General Procedure for the Preparation of 3
3.4. General Procedure for the Preparation of 4
3.5. General Procedure for the Preparation of 5a–e
3.6. General Procedure for the Preparation of 5f–i
3.7. Antiproliferative Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Gulesel, K. Isolation of matrine from the seeds of Sophora jaubertii. J. Nat. Prod. 1981, 44, 236–237. [Google Scholar]
- Nurgün, K.; Nezaket, A.; Semiha, O. Alkaloid profiles and biological activities of different Sophora jaubertii extracts. Turk J. Pharm. Sci. 2010, 7, 1–8. [Google Scholar]
- Chen, X.; Yi, C.Q.; Yang, X.Q.; Wang, X.R. Liquid chromatography of active principles in Sophora flavescens root. J. Chromatogr. B 2004, 812, 149–163. [Google Scholar] [CrossRef]
- Cho, C.H.; Chuang, C.Y.; Chen, C.F. Study of the antipyretic activity of matrine: A lupin alkaloid isolated from Sophora subprostrata. Planta Med. 1986, 52, 343–345. [Google Scholar] [CrossRef]
- Liu, J.Y.; Hu, J.H.; Zhu, Q.G.; Li, F.Q.; Wang, J.; Sun, H.J. Effect of matrine on the expression of substance P receptor and inflammatory cytokines production in human skin keratinocytes and fibrob lasts. Int. Immunopharmacol. 2007, 7, 816–823. [Google Scholar] [CrossRef]
- Nurgün, K.; Semiha, O.; Nezaket, A.; Fatma, T. Characterisation and antimicrobial activity of Sophora alopecuroides L. var. alopecuroides alkaloid extracts. Turk. J. Biol. 2011, 35, 379–385. [Google Scholar]
- Yang, Y.J.; Xiu, J.H.; Zhang, X.; Zhang, L.F.; Yan, K.; Qin, C.; Liu, J.N. Antiviral effect of matrine against human enterovirus 71. Molecules 2012, 17, 10370–10376. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, R.T.; Cui, C.; Wang, Z.H.; Tong, H.; Deng, Y.Q.; Zhang, Z.Z. Effect of oxymatrine on L929 tumor cell induced immuno suppression (Chinese). Zhong Guo Mian Yi Za Zhi 2009, 25, 216–220. [Google Scholar]
- Zhang, J.P.; Zhang, M.; Zhou, J.P.; Liu, F.T.; Zhou, B.; Xie, W.F.; Guo, C.; Zhang, C.; Qian, D.H. Antifibrotic effects of matrine on in vitro and in vivo models of liver fibrosis in rats. Acta Pharmacol. Sin. 2001, 22, 183–186. [Google Scholar]
- Luo, X.Y.; Zhang, X.M.; Gao, W.; Wu, Q.F. Studies on site of analgesic action of matrine and its mechanism (Chinese). Zhong Cao Yao 2001, 32, 41–43. [Google Scholar]
- Li, X.L.; Chu, W.M.; Liu, J.M.; Xue, X.R.; Lu, Y.J.; Shan, H.L.; Yang, B.F. Antiarrhythmic properties of long-term treatment with matrine in arrhythmic rat induced by coronary ligation. Biol. Pharm. Bull. 2009, 32, 1521–1526. [Google Scholar] [CrossRef]
- Shen, Y.Q.; Zhang, M.F. Antidiarrheal and anti-inflammatory effects of 13α-hydroxymatrine. (in Chinese). Chin. J. Pharmacol. Toxicol. 1994, 8, 206–208. [Google Scholar]
- Wang, L.S.; You, Y.J.; Wang, S.Q.; Liu, X.; Liu, B.M.; Wang, J.N.; Lin, X.; Chen, M.S.; Liang, G.; Yang, H. Synthesis, characterization and in vitro anti-tumor activities of matrine derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 4100–4102. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, C.F.; Liu, X.S.; Liu, X.S.; Jiang, J.H. In vitro anti-tumour activities of quinolizidine alkaloids derived from Sophora flavescens ait. Basic. Clin. Pharmacol. 2010, 108, 304–309. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Yu, P.F.; Liu, Q.; Liu, K.; Duan, H.Y.; Luan, G.L.; Yagasaki, K.; Zhang, G.Y. Effects of matrine against the growth of human lung cancer and hepatoma cells as well as lung cancer cell migration. Cytotechnology 2009, 59, 191–200. [Google Scholar] [CrossRef]
- Luo, C.; Zhong, H.J.; Zhu, L.M.; Wu, X.G.; Ying, J.E.; Wang, X.H.; Xia, W.; Xu, L.Q.; Zhu, Y.L.; Huang, J. Inhibition of matrine against gastric cancer cell line MNK45 growth and its anti-tumor mechanism. Mol. Biol. Rep. 2012, 39, 5459–5464. [Google Scholar] [CrossRef]
- Dai, Z.J.; Gao, J.; Ji, Z.Z.; Wang, X.J.; Ren, H.T.; Liu, X.X.; Wu, W.Y.; Kang, H.F.; Guan, H.T. Matrine induces apoptosis in gastric carcinoma cells via alteration of Fas/FasL and activation of caspase-3. J. Ethnopharmacol. 2009, 123, 91–96. [Google Scholar] [CrossRef]
- Zhang, L.P.; Jiang, J.K.; Tam, J.W.O.; Zhang, Y.; Liu, X.S.; Xu, X.R.; Liu, B.Z.; He, Y.Z. Effects of matrine on proliferation and differentiation in K-562 cells. Leukemia Res. 2001, 25, 793–800. [Google Scholar] [CrossRef]
- Zhang, S.J.; Qi, J.P.; Sun, L.B.; Cheng, B.L.; Pan, S.H.; Zhou, M.; Sun, X.Y. Matrine induces programmed cell death and regulates expression of relevant genes based on PCR array analysis in C6 glioma cells. Mol. Biol. Rep. 2009, 36, 791–799. [Google Scholar] [CrossRef]
- Liu, X.Y.; Fang, H.; Yang, Z.J.; Wang, X.Y.; Ruan, L.M.; Fang, D.R.; Ding, Y.G.; Wang, Y.N.; Zhang, Y.; Jiang, X.L.; et al. Matrine inhibits invasiveness and metastasis of human malignant melanoma cell line A375 in vitro. Int. J. Dermatol. 2008, 47, 448–456. [Google Scholar] [CrossRef]
- Zhang, L.J.; Wang, T.T; Wen, X.M.; Wei, Y.; Peng, X.C.; Li, H.; Wei, L. Effect of matrine on HeLa cell adhesion and migration. Eur. J. Pharmacol. 2007, 563, 69–76. [Google Scholar] [CrossRef]
- Gao, L.M.; Han, Y.X.; Wang, Y.P.; Li, Y.H.; Shan, Y.Q.; Li, X.; Peng, Z.G.; Bi, C.W.; Zhang, T.; Du, N.N.; et al. Design and synthesis of oxymatrine analogues overcoming drug resistance in hepatitis B virus through targeting host heat stress cognate 70. J. Med. Chem. 2011, 54, 869–876. [Google Scholar] [CrossRef]
- Hu, H.G.; Wang, S.Z.; Zhang, C.M.; Wang, L.; Ding, L.; Zhang, J.P.; Wu, Q.Y. Synthesis and in vitro inhibitory activity of matrine derivatives towards pro-inflammatory cytokines. Bioorg. Med. Chem. Lett. 2010, 20, 7537–7539. [Google Scholar] [CrossRef]
- He, L.Q.; Liu, J.; Yin, D.K.; Zhang, Y.H.; Wang, X.S. Synthesis and biological evaluation of nitric oxide-releasing matrine derivatives as anticancer agents. Chin. Chem. Lett. 2010, 21, 381–384. [Google Scholar] [CrossRef]
- Du, N.N.; Li, X.; Wang, Y.P.; Liu, F.; Liu, Y.X.; Li, C.X.; Peng, Z.G.; Gao, L.M.; Jiang, J.D.; Song, D.Q. Synthesis, structure-activity relationship and biological evaluation of novel N-substituted matrinic acid derivatives as host heat-stress cognate 70(Hsc70) down-regulator. Bioorg. Med. Chem. Lett. 2011, 21, 4732–4735. [Google Scholar] [CrossRef]
- Lo, C.Y.; Hsu, L.C.; Chen, M.S.; Lin, L.G.; Kuo, C.D.; Wu, J.Y. Synthesis and anticancer activity of a novel series of 9-O-substituted berberine derivatives: A lipophilic substitute role. Bioorg. Med. Chem. Lett. 2013, 23, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ren, L.; Liu, W.M.; Wang, J.C.; Wang, Y.L.; Tu, Q.; Xu, J.; Liu, R.; Zhang, Y.R.; Yuan, M.S.; et al. Spatiotemporally controlled and multifactor involved assay of neuronal compartment regeneration after chemical injury in anintegrated microfluidics. Anal. Chem. 2012, 84, 6444–6453. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 5a–i are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chao, F.; Wang, D.-E.; Liu, R.; Tu, Q.; Liu, J.-J.; Wang, J. Synthesis, Characterization and Activity Evaluation of Matrinic Acid Derivatives as Potential Antiproliferative Agents. Molecules 2013, 18, 5420-5433. https://doi.org/10.3390/molecules18055420
Chao F, Wang D-E, Liu R, Tu Q, Liu J-J, Wang J. Synthesis, Characterization and Activity Evaluation of Matrinic Acid Derivatives as Potential Antiproliferative Agents. Molecules. 2013; 18(5):5420-5433. https://doi.org/10.3390/molecules18055420
Chicago/Turabian StyleChao, Fan, Dong-En Wang, Rui Liu, Qin Tu, Jian-Jun Liu, and Jinyi Wang. 2013. "Synthesis, Characterization and Activity Evaluation of Matrinic Acid Derivatives as Potential Antiproliferative Agents" Molecules 18, no. 5: 5420-5433. https://doi.org/10.3390/molecules18055420




