Synthesis of a New ent-Cyclozonarone Angular Analog, and Comparison of Its Cytotoxicity and Apoptotic Effects with ent-Cyclozonarone
Abstract
:1. Introduction
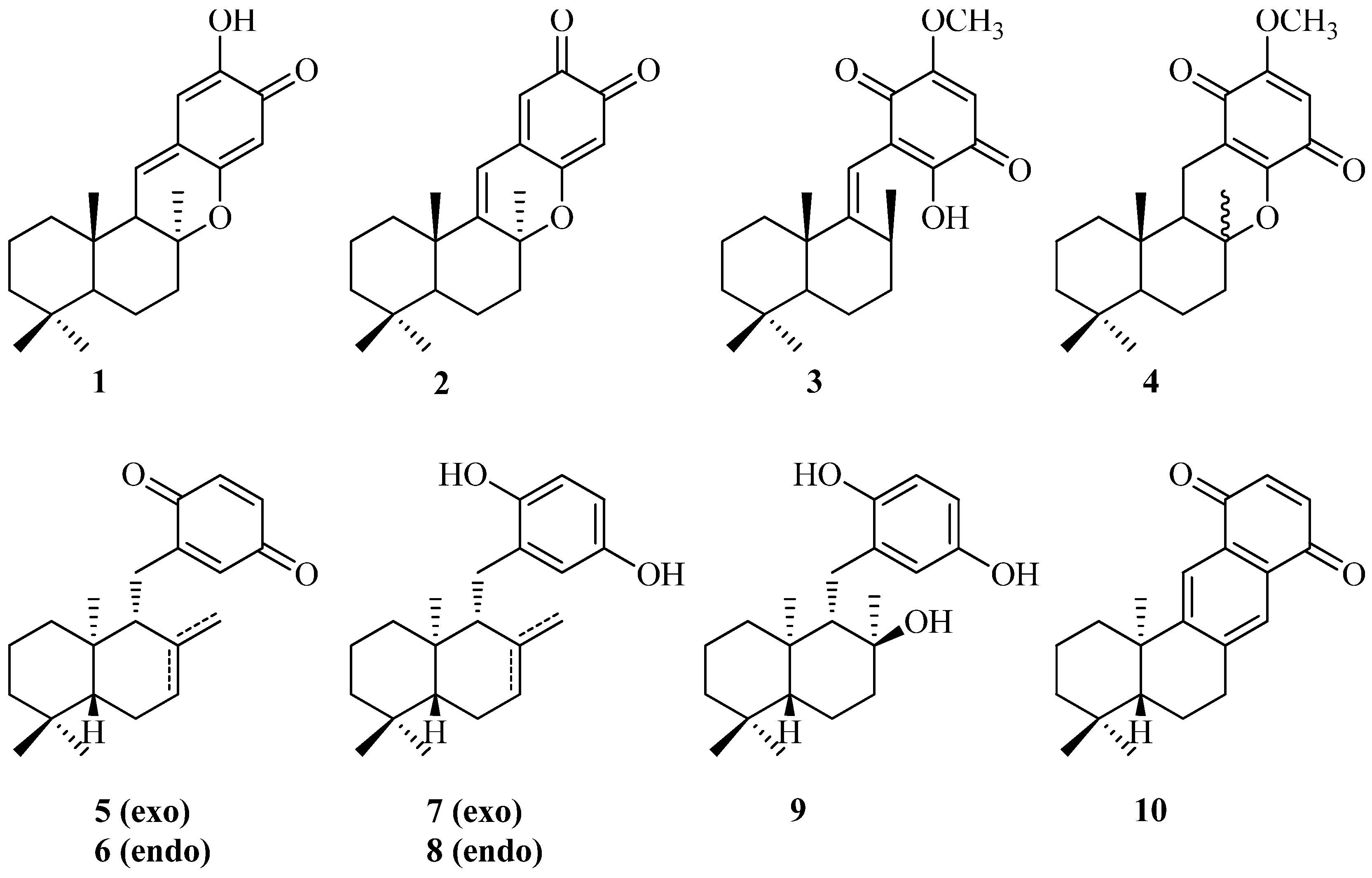
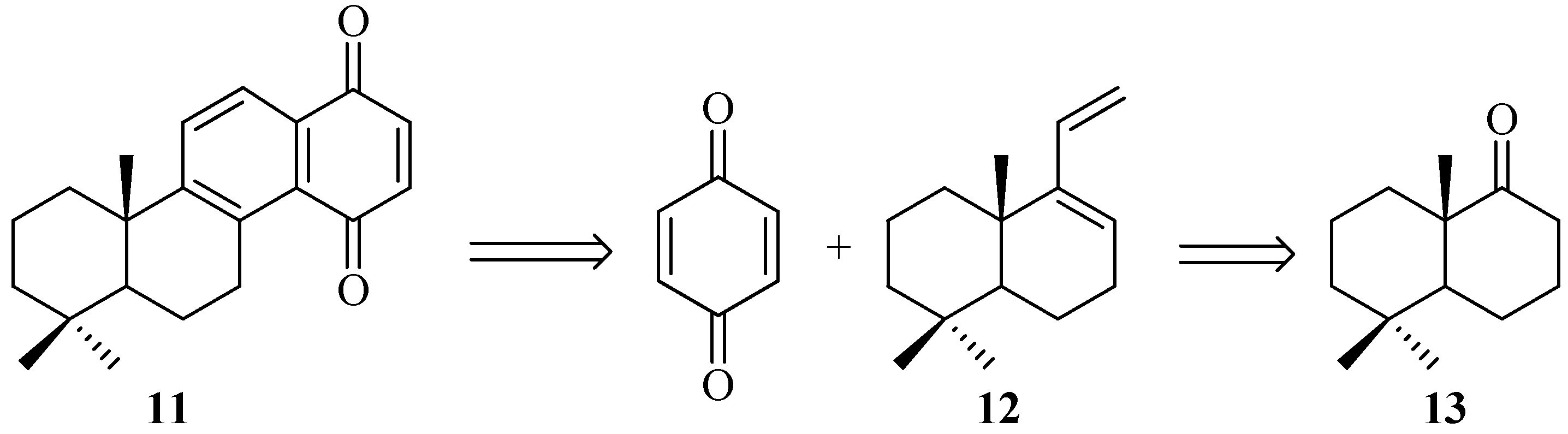
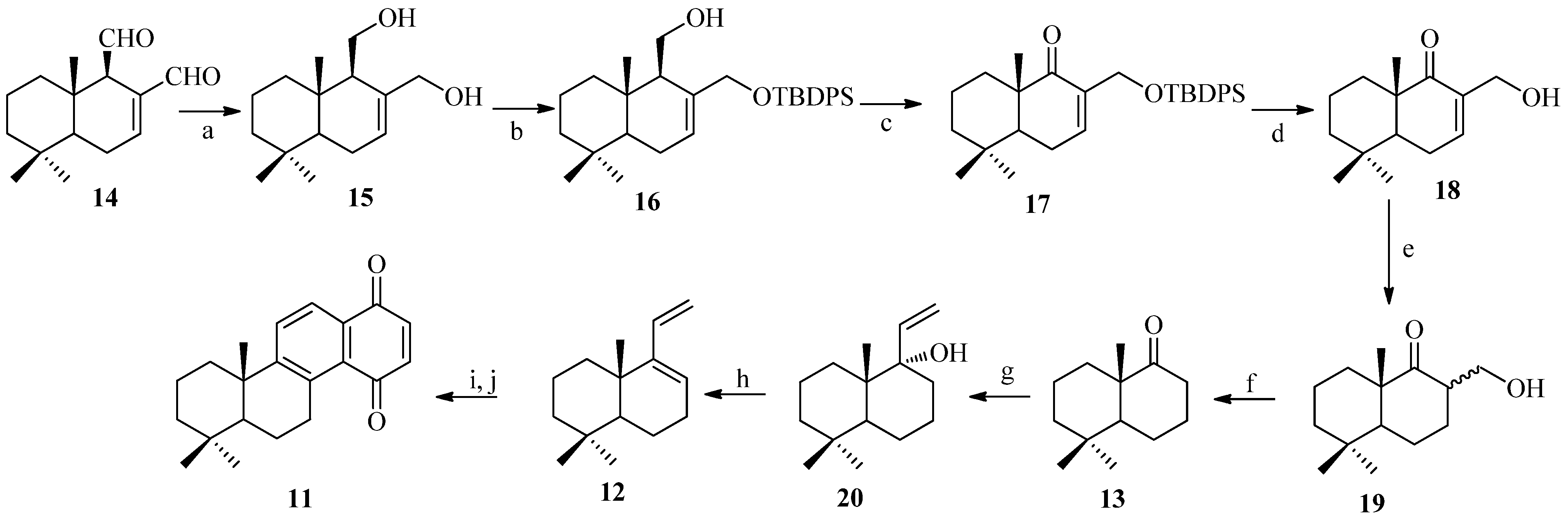
2. Results and Discussion
2.1. Chemistry
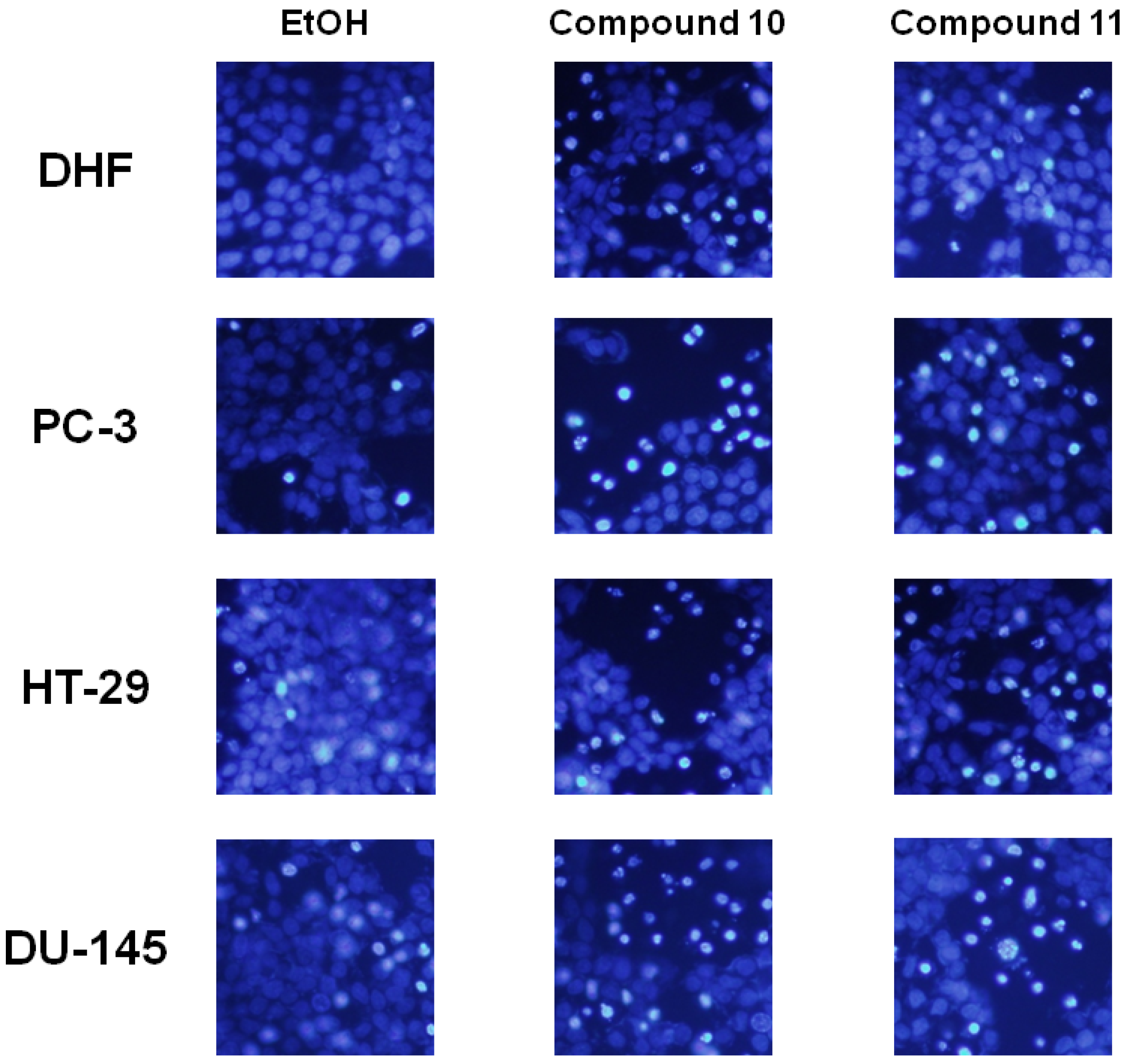
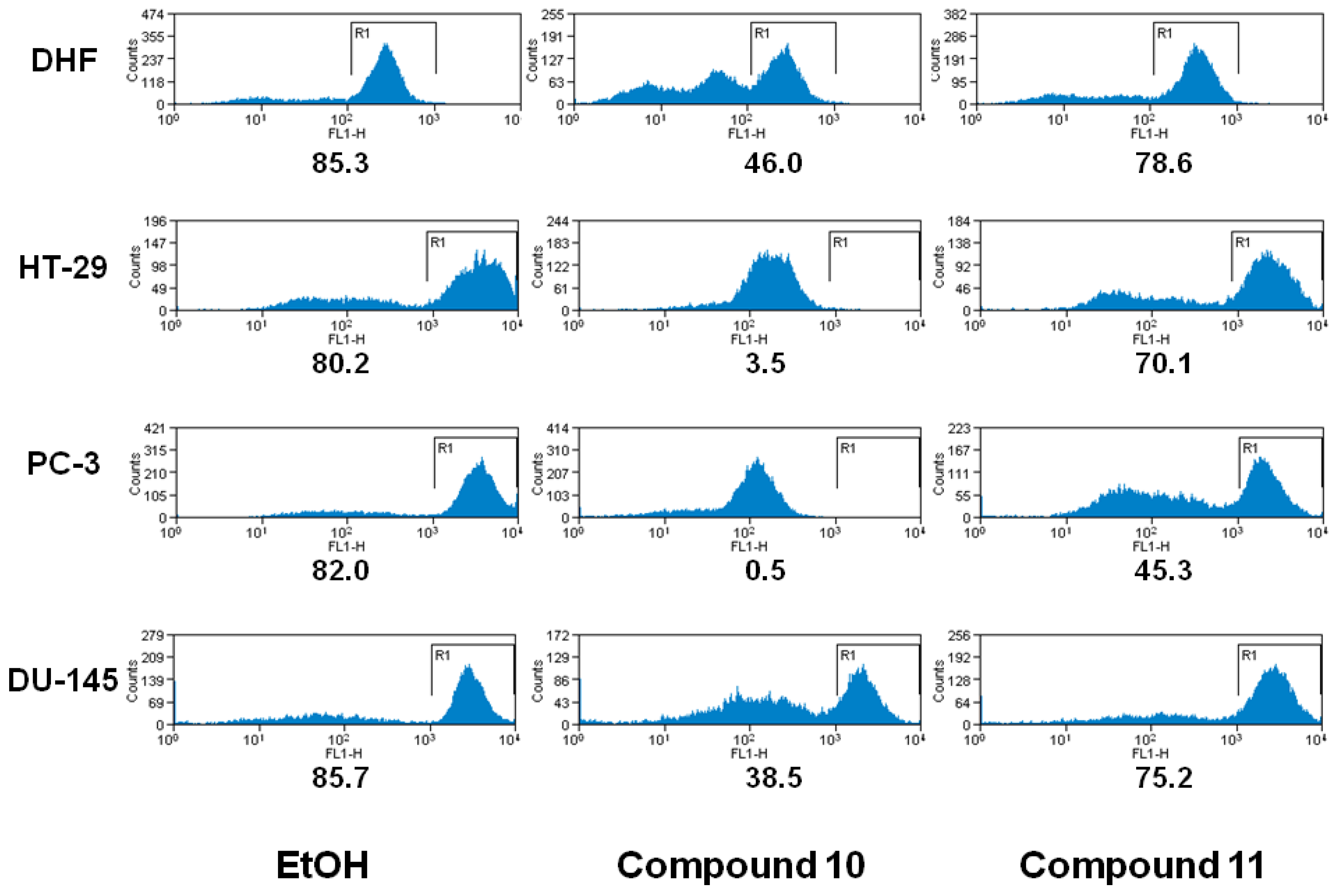
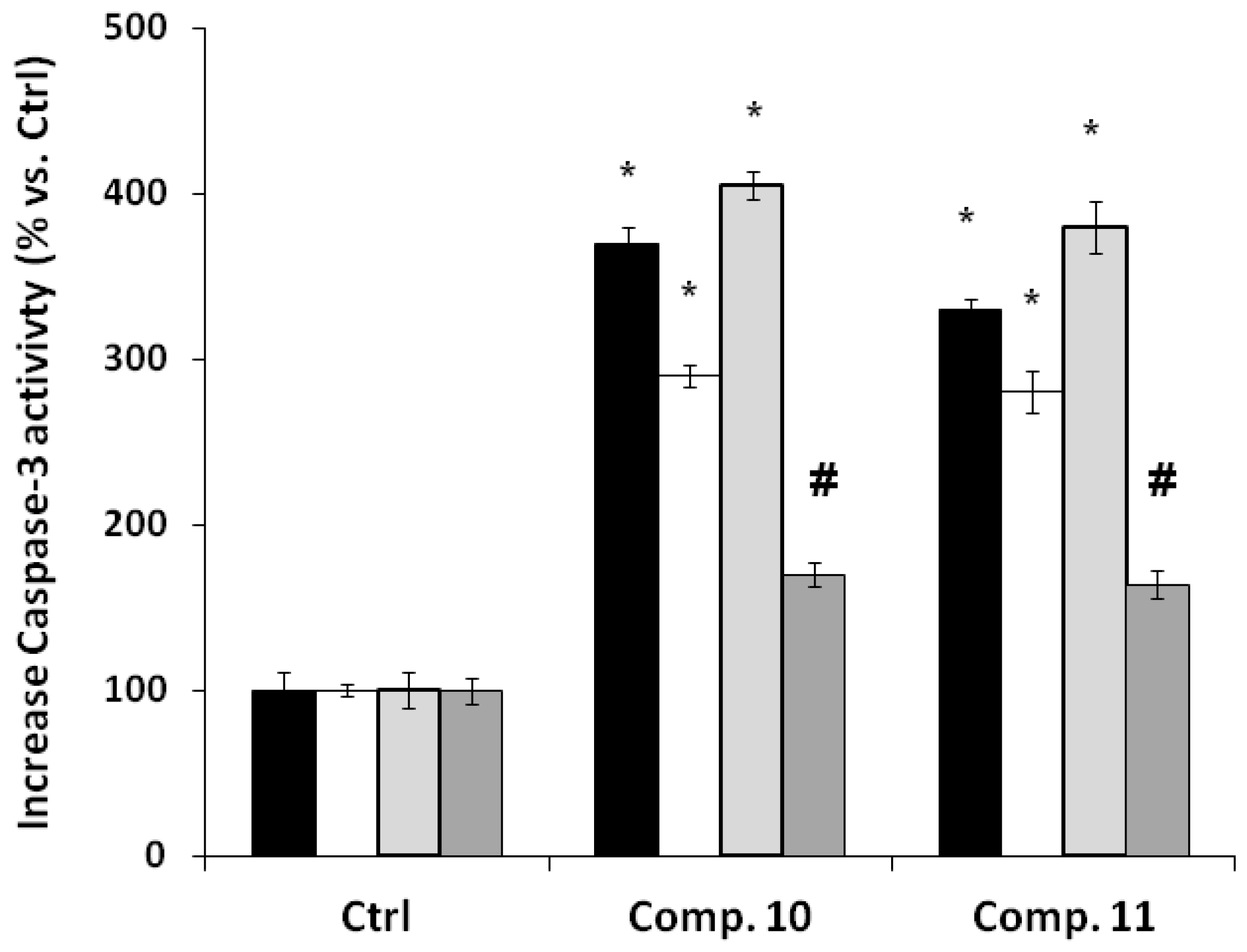
2.2. Biology
3. Experimental
3.1. General
3.2. Cell Lines
3.3. Cell Viability
3.3.1. In Vitro Growth Inhibition Assay
3.4. Morphological Assessment of Cell Apoptosis
Hoechst 33342
3.5. Analysis of Mitochondrial Membrane Permeability
3.6. Caspase 3 Activity Assay
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Gordaliza, M. Cytotoxic terpene quinones from marine sponges. Mar. Drugs 2010, 8, 2849–2870. [Google Scholar] [CrossRef] [PubMed]
- Hamann, M.T.; Scheuer, P.J.; Kellyborges, M. Biogenetically diverse, bioactive constituents of a sponge, order verongida-bromotyramines and sesquiterpene-shikimate derived metabolites. J. Org. Chem. 1993, 58, 6565–6569. [Google Scholar] [CrossRef]
- Capon, R.J.; Groves, D.R.; Urban, S.; Watson, R.G. Spongiaquinone revisited—Structural and stereochemical studies on marine sesquiterpene quinones from a southern australian marine sponge,spongia sp. Aust. J. Chem. 1993, 46, 1245–1253. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Murphy, P.; Warren, R.; Wells, R.; Blount, J. New quinones from a dictyoceratid sponge. Aust. J. Chem. 1978, 31, 2685–2697. [Google Scholar] [CrossRef]
- Kurata, K.; Taniguchi, K.; Suzuki, M. Cyclozonarone, a sesquiterpene-substituted benzoquinone derivative from the brown alga Dictyopteris undulata. Phytochemistry 1996, 41, 749–752. [Google Scholar] [CrossRef]
- Laube, T.; Beil, W.; Seifert, K. Total synthesis of two 12-nordrimanes and the pharmacological active sesquiterpene hydroquinone yahazunol. Tetrahedron 2005, 61, 1141–1148. [Google Scholar] [CrossRef]
- Cuellar, M.A.; Salas, C.; Cortes, M.J.; Morello, A.; Maya, J.D.; Preite, M.D. Synthesis and in vitro trypanocide activity of several polycyclic drimane-quinone derivatives. Bioorg. Med. Chem. 2003, 11, 2489–2497. [Google Scholar] [CrossRef]
- Delgado, V.; Armstrong, V.; Cortes, M.; Barrero, A.F. Synthesis of racemic and chiral albicanol, albicanyl acetate and cyclozonarone: Cytotoxic activity of ent-cyclozonarone. J. Braz. Chem. Soc. 2008, 19, 1258–1263. [Google Scholar] [CrossRef]
- Cortes, M.; Valderrama, J.A.; Cuellar, M.; Armstrong, V.; Preite, M. Synthesis of (+)-cyclozonarone and the absolute configuration of naturally occurring (−)-cyclozonarone. J. Nat. Prod. 2001, 64, 348–349. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.; Matthes, B.; Seifert, K. Total synthesis of the marine sesquiterpene quinone (−)-cyclozonarone. Tetrahedron Lett. 2001, 42, 8151–8152. [Google Scholar] [CrossRef]
- Henry, T.R.; Wallace, K.B. Differential mechanisms of cell killing by redox cycling and arylating quinones. Arch. Toxicol. 1996, 70, 482–489. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.J. Molecular mechanisms of quinone cytotoxicity. Chem. Biol. Interac. 1991, 80, 1–41. [Google Scholar] [CrossRef]
- Reddy, L.; Odhav, B.; Bhoola, K.D. Natural products for cancer prevention: A global perspective. Pharmacol. Ther. 2003, 99, 1–13. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Witzig, T.E.; Adjei, A.A. Targeting apoptosis pathways in cancer therapy. CA Cancer J. Clin. 2005, 55, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, A.H. Apoptosis: An overview. Br. Med. Bull. 1997, 53, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Makin, G.; Hickman, J.A. Apoptosis and cancer chemotherapy. Cell Tissue Res. 2000, 301, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, A.; Ghadiri, A.; Dessauge, F.; Duhamel, M.; Rebollo, M.P.; Alvarez-Franco, F.; Rebollo, A. Modulating apoptosis as a target for effective therapy. Mol. Immunol. 2006, 43, 1065–1079. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Emi, M.; Tanabe, K. Role of mitochondria as the gardens of cell death. Cancer Chem. Pharmacol. 2006, 57, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, C.A.; Linden, R. Programmed cell deaths—Apoptosis and alternative deathstyles. Eur. J. Biochem. 2004, 271, 1638–1650. [Google Scholar] [CrossRef] [PubMed]
- Lemasters, J.J.; Nieminen, A.L.; Qian, T.; Trost, L.C.; Elmore, S.P.; Nishimura, Y.; Crowe, R.A.; Cascio, W.E.; Bradham, C.A.; Brenner, D.A.; et al. The mitochondrial permeability transition in cell death: A common mechanism in necrosis, apoptosis and autophagy. Biochim. Biophys. Acta 1998, 1366, 177–196. [Google Scholar] [CrossRef]
- Reed, J.C.; Tomaselli, K.J. Drug discovery opportunities from apoptosis research. Curr. Opin. Biotech. 2000, 11, 586–592. [Google Scholar] [CrossRef]
- Mattson, M.P.; Kroemer, G. Mitochondria in cell death: Novel targets for neuroprotection and cardioprotection. Trends Mol. Med. 2003, 9, 196–205. [Google Scholar] [CrossRef]
- Gordaliza, M. Synthetic strategies to terpene quinones/hydroquinones. Mar. Drugs 2012, 10, 358–402. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lam, M.S.; Birkeland, A.; Riffel, A.; Montana, L.; Sullivan, M.E.; Post, J.M. Cell-based assays for profiling activity and safety properties of cancer drugs. J. Pharmacol. Toxicol. Methods 2006, 54, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Kamb, A.; Lassota, P. Disease models of cancer: Apoptosis. Drug Discov. Today 2004, 1, 31–36. [Google Scholar] [CrossRef]
- Cuellar, M.A.; Moreno, L.E.; Preite, M.D. Regioselective oxidative fragmentation of drimanic terpene alcohols: A short, easy and efficient access to natural and synthetic 11-nordrimane terpene derivatives. ARKIVOC 2003, 169–177. [Google Scholar]
- Benites, J.; Preite, M.D.; Cortes, M. Conversion of (+)-confertifolin into 11,12-bisnordriman-9-one and (+)-8αH, 9αH-11,12-diacetoxydrimane. Syn. Commun. 2001, 31, 1347–1354. [Google Scholar] [CrossRef]
- Coisne, J.M.; Pecher, J. A terpene synthesis intermediate: (±)-5-ethenyl-1, 1, 4aβ-trimethyl-1, 2, 3, 4, 4a, 7, 8, 8aα-octahydronaphthalene. Bull. Soc. Chim. Belg. 1980, 89, 551–557. [Google Scholar] [CrossRef]
- Emaus, R.K.; Grunwald, R.; Lemasters, J.J. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: Spectral and metabolic properties. Biochim. Biophys. Acta 1986, 850, 436–448. [Google Scholar] [CrossRef]
- Day, T.W.; Wu, C.H.; Safa, A.R. Etoposide induces protein kinase Cδ- and caspase-3-dependent apoptosis in neuroblastoma cancer cells. Mol. Pharmacol. 2009, 76, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.

| Compound | DU-145 | PC-3 | HT-29 | DHF |
|---|---|---|---|---|
| (+)-10 | 6.4 ± 0.8 | 9.5 ± 1.2 | 25.0 ± 0.9 | 48.0 ± 2.3 |
| 11 | 13.3 ± 1.4 | 14.9 ± 1.8 | 45.0 ± 0.5 | 91.0 ± 7.8 |
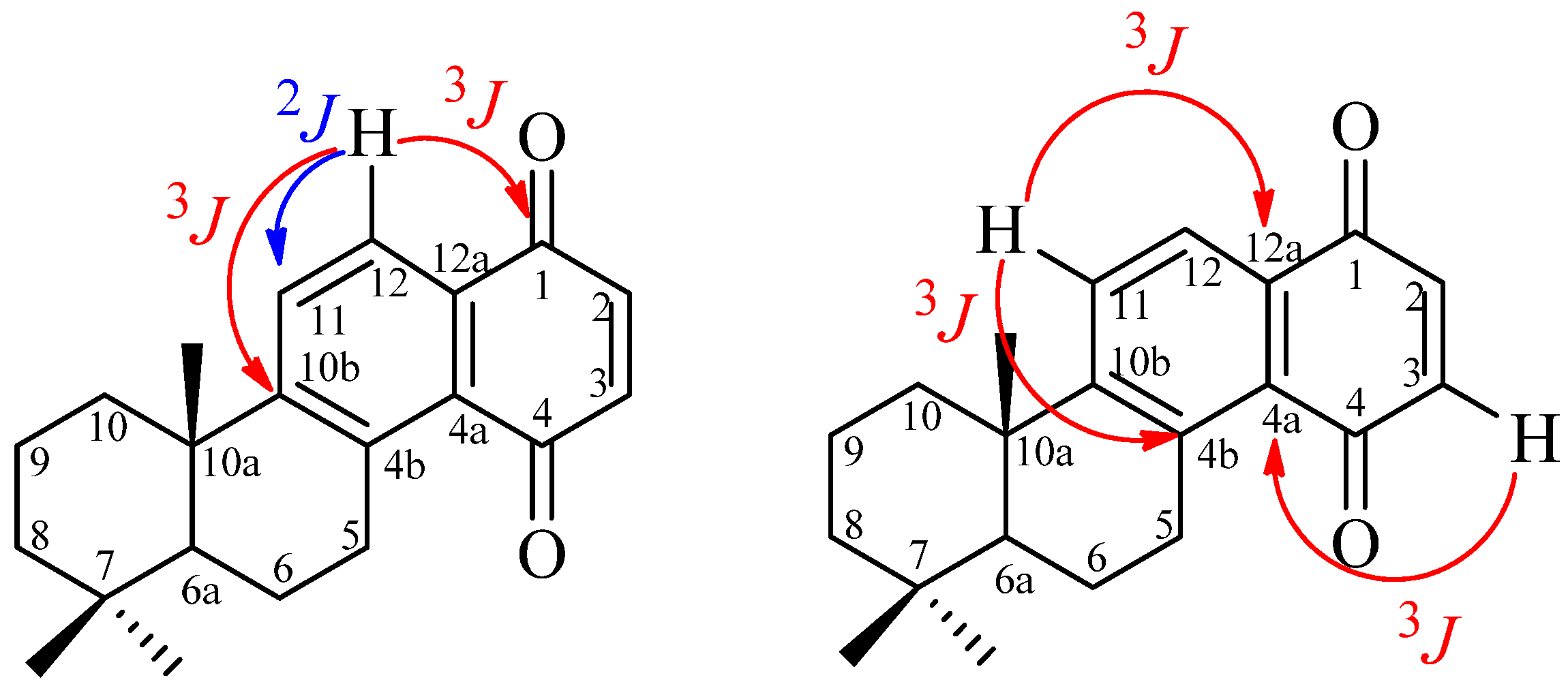
| Position | 1H-NMR | 13C-NMR |
|---|---|---|
| 1 | __ | 185.6 |
| 2 | 6.87 (1H, s) | 141.1 * |
| 3 | 6.87 (1H, s) | 136.2 * |
| 4 | __ | 187.7 |
| 4a | __ | 129.2 |
| 4b | __ | 139.9 |
| 5 | 3.52 (1H, ddd, 6.0, 6.0, 19.7); 3.29 (1H, ddd, 8.1, 11.2, 19.7) | 30.2 |
| 6 | 1.69 (2H, m) | 19.3 |
| 6a | 1.34 (1H, dd, 2.01, 12.4) | 49.0 |
| 7 | __ | 29.7 |
| 8 | 1.53 (1H, bs, 13.2); 1.3 (1H, m) | 41.2 |
| 9 | 2.02 (1H, m); 1.65 (1H, m) | 18.8 |
| 10 | 2.34 (1H, bs, 12.7); 1.41 (1H, dd, 3.7, 12.9) | 39.2 |
| 10a | __ | 33.4 |
| 10b | __ | 158.3 |
| 11 | 7.72 (1H, d, 8.3) | 130.2 |
| 12 | 7.98 (1H, d, 8.3) | 124.9 |
| 12a | __ | 131.3 |
| 7-α-Me | 1.00 (3H, s) | 33.1 |
| 7-β-Me | 0.97 (3H, s) | 24.7 |
| 10a-Me | 1.27 (3H, s) | 21.6 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sobarzo, N.Q.; Venegas, I.M.; Sánchez, C.S.; Catalán, L.E.; Rojas, C.C.; Valdivia, V.U.; García, J.V.; Fritis, M.C. Synthesis of a New ent-Cyclozonarone Angular Analog, and Comparison of Its Cytotoxicity and Apoptotic Effects with ent-Cyclozonarone. Molecules 2013, 18, 5517-5530. https://doi.org/10.3390/molecules18055517
Sobarzo NQ, Venegas IM, Sánchez CS, Catalán LE, Rojas CC, Valdivia VU, García JV, Fritis MC. Synthesis of a New ent-Cyclozonarone Angular Analog, and Comparison of Its Cytotoxicity and Apoptotic Effects with ent-Cyclozonarone. Molecules. 2013; 18(5):5517-5530. https://doi.org/10.3390/molecules18055517
Chicago/Turabian StyleSobarzo, Natalia Quiñones, Iván Montenegro Venegas, Cristian Salas Sánchez, Luis Espinoza Catalán, Cristóbal Carrasco Rojas, Valentina Ulloa Valdivia, Joan Villena García, and Mauricio Cuellar Fritis. 2013. "Synthesis of a New ent-Cyclozonarone Angular Analog, and Comparison of Its Cytotoxicity and Apoptotic Effects with ent-Cyclozonarone" Molecules 18, no. 5: 5517-5530. https://doi.org/10.3390/molecules18055517




