Effects of Chirality on the Antifungal Potency of Methylated Succinimides Obtained by Aspergillus fumigatus Biotransformations. Comparison with Racemic Ones
Abstract
:1. Introduction
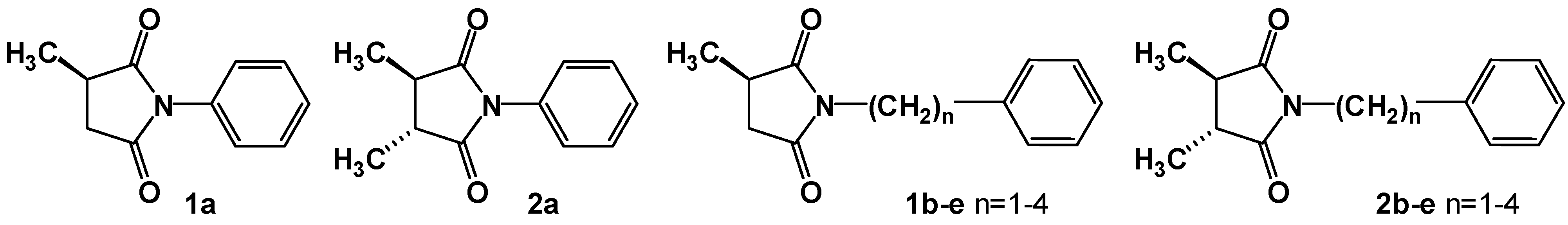

2. Results and Discussion

| Substrate | R1 | R2 | Product | % ee | % Conv. |
|---|---|---|---|---|---|
| 11 | CH3 | H | (R)-(+)-3 | 97 | 93 |
| 12 | OCH3 | H | (R)-(+)-4 | >99 | 97 |
| 13 | NO2 | H | (R)-(+)-5 | 98 | 95 |
| 14 | F | H | (R)-(+)-6 | 98 | 99 |
| 15 | Cl | H | (R)-(+)-7 | 97 | 91 |
| 16 | Br | H | (R)-(+)-8 | 98 | 81 |
| 17 | H | CH3 | (R)-(+)-9 | 98 | 65 |
| 18 | F | F | (R)-(+)-10 | 99 | 55 |
| 28 * | H | H | (R)-(+)-1a | >99 | 99 |
| Type | n | Conf. | Ca1 | Ct2 | Ck3 | Cg4 | Cp5 | Cl6 | Sc7 | Cn8 | Mg9 | Tr10 | Tm11 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1a | A | 0 | - | R- | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 31.3 | 62.5 |
| rac- | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 62.5 | 125 | 62.5 | |||||
| ER | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | - | 4 | - | |||||
| 1b | A | 1 | - | R- | 15.6 | 31.3 | 62.5 | 62.5 | 62.5 | 31.3 | 62.5 | 15.6 | 125 | 62.5 | 62.5 | |
| rac- | 62.5 | 125 | 125 | 125 | 125 | 125 | 125 | 62.5 | 250 | 125 | 250 | |||||
| ER | 4 | 4 | 2 | 2 | 2 | 4 | 2 | 4 | 2 | 2 | 4 | |||||
| 1c | A | 2 | - | R- | 31.3 | 62.5 | 125 | 62.5 | 31.3 | 62.5 | 62.5 | 62.5 | 125 | 62.5 | 31.3 | |
| rac- | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 62.5 | 62.5 | |||||
| ER | 4 | 2 | - | 2 | 4 | 2 | 2 | 2 | - | - | 2 | |||||
| 1d | A | 3 | - | R- | 62.5 | 125 | 125 | 62.5 | 125 | 125 | 125 | 62.5 | 62.5 | 125 | 62.5 | |
| rac- | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 250 | 250 | |||||
| ER | 2 | - | - | - | - | - | - | 2 | 2 | 2 | 4 | |||||
| 1e | A | 4 | - | R- | 31.3 | 31.3 | 62.5 | 125 | 62.5 | 125 | 62.5 | 31.3 | 125 | 62.5 | 31.3 | |
| rac- | 62.5 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 250 | 125 | 125 | |||||
| ER | 2 | 4 | 2 | - | 2 | - | 2 | 4 | 2 | 2 | 4 | |||||
| 2 | 2a | B | 0 | - | 3R,4R- | 31.3 | 31.3 | 62.5 | 62.5 | 31.3 | 62.5 | 62.5 | 62.5 | 125 | 125 | 62.5 |
| rac- | 125 | 62.5 | 62.5 | 125 | 62.5 | 62.5 | 125 | 125 | 125 | 125 | 125 | |||||
| ER | 4 | 2 | - | 2 | 2 | - | 2 | 2 | - | - | 2 | |||||
| 2b | B | 1 | - | 3R,4R- | 31.3 | 62.5 | 62.5 | 125 | 62.5 | 62.5 | 125 | 62.5 | 62.5 | 62.5 | 31.3 | |
| rac- | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 62.5 | 62.5 | 125 | |||||
| ER | 4 | 2 | 2 | - | 2 | 2 | - | 2 | - | - | 4 | |||||
| 2c | B | 2 | - | 3R,4R- | 15.6 | 125 | 125 | 62.5 | 62.5 | 62.5 | 125 | 62.5 | 125 | 125 | 62.5 | |
| rac- | 125 | 125 | 250 | 125 | 250 | 125 | 125 | 125 | 250 | 250 | 62.5 | |||||
| ER | 8 | - | 2 | 2 | 4 | 2 | - | 2 | 2 | 2 | - | |||||
| 2d | B | 3 | - | 3R,4R- | 31.3 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 31.3 | 31.3 | |
| rac- | 62.5 | 125 | 125 | 125 | 125 | 250 | 125 | 125 | 125 | 62.5 | 125 | |||||
| ER | 2 | 2 | 2 | 2 | 2 | 4 | 2 | 2 | 2 | 2 | 4 | |||||
| 2e | B | 4 | - | 3R,4R- | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 31.3 | 62.5 | 62.5 | 62.5 | |
| rac- | 125 | 125 | 62.5 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | |||||
| ER | 2 | 2 | - | 2 | 2 | 2 | 2 | 4 | 2 | 2 | 2 | |||||
| 3 | C | 0 | 4'-CH3 | R- | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 125 | 125 | 125 | |
| rac- | 250 | 250 | 125 | 125 | 250 | 250 | 125 | 62.5 | 250 | 250 | 250 | |||||
| ER | 4 | 4 | 2 | 2 | 4 | 4 | 2 | - | 2 | 2 | 2 | |||||
| 4 | C | 0 | 4'-OMe | R- | 125 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 125 | 62.5 | 125 | 125 | 125 | |
| rac- | 250 | 125 | 125 | 125 | 125 | 125 | 125 | 62.5 | 250 | 250 | 250 | |||||
| ER | 2 | 2 | 2 | 2 | 2 | 2 | - | - | 2 | 2 | 2 | |||||
| 5 | C | 0 | 4'-NO2 | R- | 62.5 | 125 | 62.5 | 125 | 62.5 | 125 | 125 | 62.5 | 125 | 125 | 125 | |
| rac- | 250 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 250 | 250 | 250 | |||||
| ER | 4 | - | 2 | - | 2 | - | - | 2 | 2 | 2 | 2 | |||||
| 6 | C | 0 | 4'-F | R- | 62.5 | 62.5 | 31.3 | 62.5 | 31.3 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | |
| rac- | 250 | 250 | 125 | 125 | 250 | 250 | 125 | 62.5 | 125 | 125 | 125 | |||||
| ER | 4 | 4 | 4 | 2 | 8 | 4 | 2 | - | 2 | 2 | 2 | |||||
| 7 | C | 0 | 4'-Cl | R- | 125 | 62.5 | 125 | 62.5 | 125 | 62.5 | 62.5 | 31.3 | 125 | 125 | 125 | |
| rac- | 250 | 250 | 125 | 250 | 250 | 250 | 125 | 125 | 250 | 250 | 250 | |||||
| ER | 2 | 4 | - | 4 | 2 | 4 | 2 | 4 | 2 | 2 | 2 | |||||
| 8 | C | 0 | 4'-Br | R- | 125 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 125 | 125 | |
| rac- | 250 | 250 | 125 | 125 | 250 | 250 | 125 | 125 | 125 | 250 | 125 | |||||
| ER | 2 | 4 | 2 | 2 | 4 | 4 | 2 | 2 | 2 | 2 | - | |||||
| 9 | C | 0 | 2'-CH3 | R- | 125 | 125 | 62.5 | 125 | 125 | 125 | 125 | 62.5 | 125 | 125 | 125 | |
| rac- | 250 | 125 | 125 | 125 | 250 | 125 | 125 | 125 | 250 | 250 | 250 | |||||
| ER | 2 | - | 2 | - | 2 | - | - | 2 | 2 | 2 | 2 | |||||
| 10 | C | 0 | 2',4'-F2 | R- | 62.5 | 62.5 | 31.3 | 62.5 | 31.3 | 125 | 62.5 | 62.5 | 62.5 | 62.5 | 125 | |
| rac- | 250 | 250 | 125 | 125 | 250 | 250 | 125 | 125 | 125 | 250 | 250 | |||||
| ER | 4 | 4 | 4 | 2 | 8 | 2 | 2 | 2 | 2 | 4 | 2 | |||||
| AmphotericinB | 0.97 | 0.48 | 0.49 | 0.49 | 0.98 | 0.98 | 0.48 | 0.24 | 0.12 | 0.06 | 0.06 | |||||
| Ketoconazole | 0.48 | 0.12 | 62.5 | 1.95 | 0.98 | 0.98 | 0.48 | 0.24 | 0.06 | 0.03 | 0.03 | |||||
| Terbinafine | - | - | - | - | - | - | - | - | 0.03 | 0.01 | 0.03 | |||||
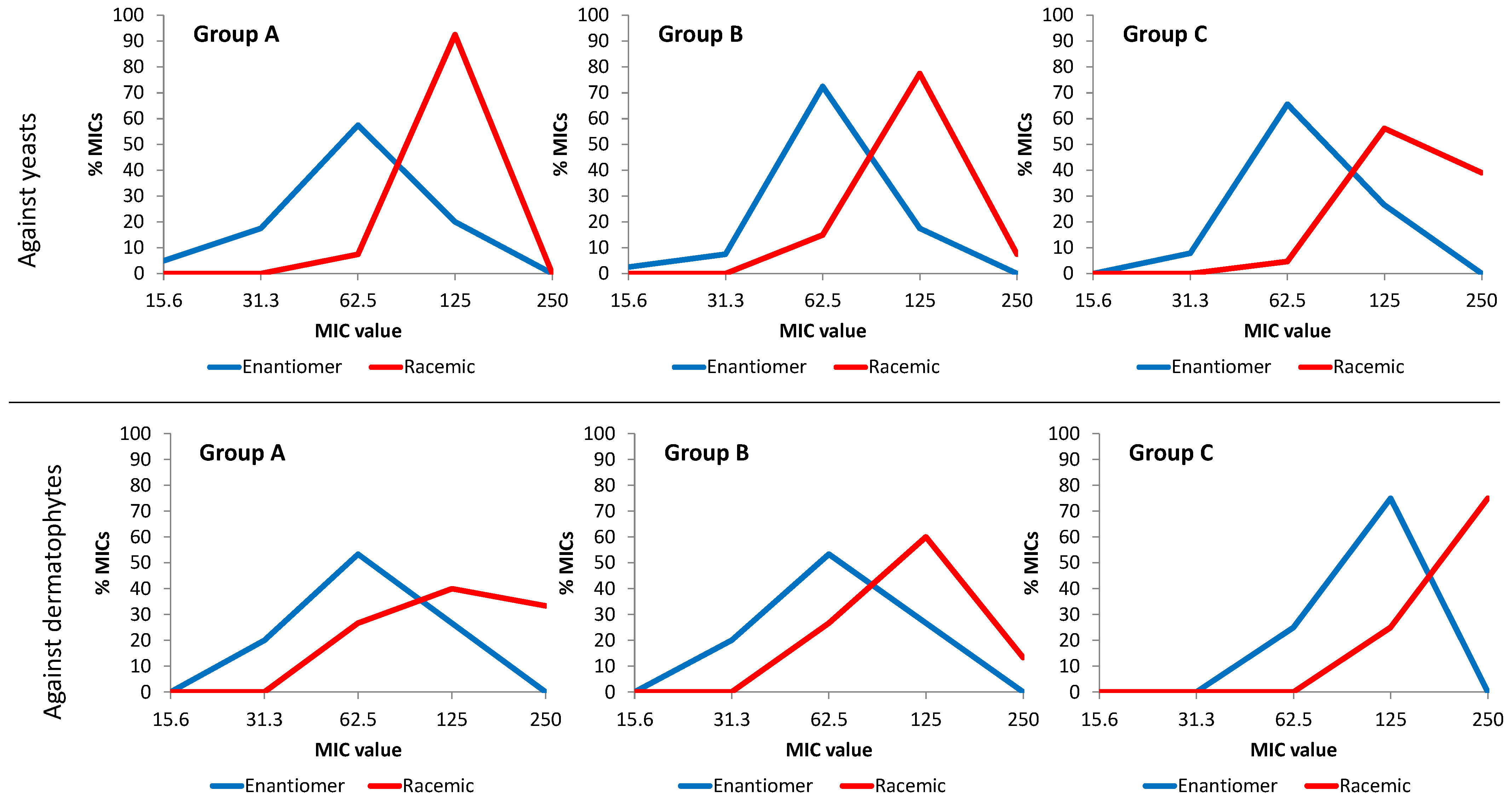

3. Experimental
3.1. General
3.2. Synthesis
3.3. Biotransformations
3.4. Antifungal Susceptibility Testing
3.5. Statistical Tests
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Groutas, W.C.; Brubaker, M.J.; Chong, L.S.; Venkataraman, R.; Huang, H.; Epp, J.B.; Kuang, R. Design, synthesis and biological evaluation of succinimide derivatives as potential mechanism-based inhibitors of human leukocyte elastase, cathepsin G and proteinas. Bioorg. Med. Chem. 1995, 3, 375–381. [Google Scholar]
- Curtin, M.L.; Garland, R.B.; Heyman, H.R.; Frey, R.R.; Michaelides, M.R.; Li, J.; Glaser, K.; Marcotte, P.; Davidsen, S.K. Succinimide hydroxamic acids as potent inhibitors of histone deacetylase (HDAC). Bioorg. Med. Chem. Lett. 2002, 12, 2919–2923. [Google Scholar]
- Ibnusaud, I.; Thomas, G. Biologically interesting chiral 3,4-disubstituted pyrrolidines from optically active hydroxycitric acid lactones. Tetrahedron Lett. 2003, 44, 1247–1249. [Google Scholar]
- Isaka, M.; Rugseree, N.; Maithip, P.; Kongsaeree, P.; Prabpai, S.; Thebtaranonth, Y. Hirsutellones A–E, antimycobacterial alkaloids from the insect pathogenic fungus Hirsutella nivea BCC 2594. Tetrahedron 2005, 61, 5577–5583. [Google Scholar]
- Uddin, J.; Ueda, K.; Siwu, E.R.; Kita, M.; Uemura, D. Cytotoxic labdane alkaloids from an ascidian Lissoclinum sp.: Isolation, structure elucidation, and structure-activity relationship. Bioorg. Med. Chem. 2006, 14, 6954–6961. [Google Scholar]
- Chauhan, P.; Jasneet, K.; Chimni, S. Asymmetric organocatalytic addition reactions of maleimides: A promising approach towards the synthesis of chiral succinimide derivatives. Chem. Asian J. 2013, 8, 328–346. [Google Scholar]
- Fredenhagen, A.; Tamura, S.Y.; Kenny, P.T.; Komura, H.; Naya, Y.; Nakanishi, K.; Nishiyama, K.; Sugiura, M.; Kita, H. Andrimid, a new peptide antibiotic produced by an intracellular bacterial symbiont isolated from a brown planthopper. J. Am. Chem. Soc. 1987, 109, 4409–4411. [Google Scholar]
- Ohshima, T.; Tadaoka, H.; Hori, K.; Sayo, N.; Mashima, K. Highly enantio- and s-trans C=C bond selective catalytic hydrogenation of cyclic enones: Alternative synthesis of (−)-menthol. Chem. Eur. J. 2008, 14, 2060–2066. [Google Scholar]
- Borges, K.B.; Borges, W.D.; Durán-Patrón, R.; Pupo, M.T.; Bonato, P.S.; Collado, I.G. Stereoselective biotransformations using fungi as biocatalysts. Tetrahedron Asymmetry 2009, 20, 385–397. [Google Scholar]
- Valadez-Blanco, R.; Livingston, A.G. Enantioselective whole-cell biotransformation of acetophenone to S-phenylethanol by Rhodotorula glutinis: Part I. Product formation kinetics and feeding strategies in aqueous media. Biochem. Eng. J. 2009, 46, 44–53. [Google Scholar]
- Patel, R.N. Biocatalysis: Synthesis of key intermediates for development of pharmaceuticals. ACS Catal. 2011, 1, 1056–1074. [Google Scholar]
- Kasprzyk-Hordern, B. Pharmacologically active compounds in the environment and their chirality. Chem. Soc. Rev. 2010, 39, 4466–4503. [Google Scholar]
- Sortino, M.; Cechinel Filho, V.; Zacchino, S. Highly enantioselective reduction of the C-C double bond of N-phenyl-2-methyl-and N-phenyl-2,3-dimethyl-maleimides by fungal strains. Tetrahedron Asymmetry 2009, 20, 1106–1108. [Google Scholar] [CrossRef]
- Sortino, M.; Zacchino, S. Efficient asymmetric hydrogenation of the C-C double bond of 2-methyl-and 2,3-dimethyl-N-phenylalkylmaleimides by Aspergillus fumigatus. Tetrahedron Asymmetry 2010, 21, 535–539. [Google Scholar]
- Hutt, A.G.; O'Grady, J. Drug chirality: A consideration of the significance of the stereochemistry of antimicrobial agents. J. Antimicrob. Chemother. 1996, 37, 7–32. [Google Scholar]
- Sortino, M.; Zacchino, S. Highly enantiomeric hydrogenation of C-C double bound of methylated N-phenyl and N-phenylalkylmaleimides by Aspergillus fumigatus. In Practical Methods for Biocatalysis and Biotransformations 2; Whittall, J., Sutton, P., Eds.; Wiley & Sons: New York, NY, USA, 2012; pp. 108–114. [Google Scholar]
- Balenović, K.; Bregant, N. Correlation of the configurations of α-methyl-β-alanine and methylsuccinic acid. J. Chem. Soc. 1965, 5131–5132. [Google Scholar]
- Hegazy, M.E.; Shishido, K.; Hirata, T. Asymmetric hydrogenation of the C-C double bond of 1-and 1,2-methylated maleimides with cultured suspension cells of Marchantia polymorpha. Tetrahedron Asymmetry 2006, 17, 1859–1862. [Google Scholar]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar]
- Kontoyiannis, D.P.; Mantadakis, E.; Samonis, G. Systemic mycoses in the immunocompromised host: An update in antifungal therapy. J. Hosp. Inf. 2003, 53, 243–258. [Google Scholar] [CrossRef]
- Singh, N. Treatment of opportunistic mycoses: How long is long enough? Lancet Infect. Dis. 2003, 3, 703–708. [Google Scholar]
- Weitzman, I.; Summerbell, R.C. The dermatophytes. Clin. Microbiol. Rev. 1995, 8, 240–259. [Google Scholar]
- CLSI (Clinical and Laboratory Standards Institute) document. In Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard M27-A3, 3rd ed.; CLSI: Wayne, PA, USA, 2008; pp. 1–25.
- CLSI (Clinical and Laboratory Standards Institute) document. In Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard M38-A2, 2nd ed.; CLSI: Wayne, PA, USA, 2008; pp. 1–35.
- Stokes, M.; Davis, Ch.; Koch, G. Categorical Data Analysis Using the SAS System, 2nd ed.; SAS Institute: Cary, NC, USA, 2000. [Google Scholar]
- Alcaide, B.; Almendros, P.; Cabrero, G.; Ruiz, M.P. Organocatalytic ring expansion of β-lactams to γ-lactams through a novel N1-C4 bond cleavage. Direct synthesis of enantiopure succinimide derivatives. Org. Lett. 2005, 7, 3981–3984. [Google Scholar] [CrossRef]
- Sample Availability: Samples of compounds 11–18 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sortino, M.; Postigo, A.; Zacchino, S. Effects of Chirality on the Antifungal Potency of Methylated Succinimides Obtained by Aspergillus fumigatus Biotransformations. Comparison with Racemic Ones. Molecules 2013, 18, 5669-5683. https://doi.org/10.3390/molecules18055669
Sortino M, Postigo A, Zacchino S. Effects of Chirality on the Antifungal Potency of Methylated Succinimides Obtained by Aspergillus fumigatus Biotransformations. Comparison with Racemic Ones. Molecules. 2013; 18(5):5669-5683. https://doi.org/10.3390/molecules18055669
Chicago/Turabian StyleSortino, Maximiliano, Agustina Postigo, and Susana Zacchino. 2013. "Effects of Chirality on the Antifungal Potency of Methylated Succinimides Obtained by Aspergillus fumigatus Biotransformations. Comparison with Racemic Ones" Molecules 18, no. 5: 5669-5683. https://doi.org/10.3390/molecules18055669







