Identification of Insecticidal Constituents of the Essential Oil of Acorus calamus Rhizomes against Liposcelis bostrychophila Badonnel
Abstract
:1. Introduction
2. Results and Discussion
2.1. Essential Oil Chemical Composition
| RI * | Compound | Composition, % | |
|---|---|---|---|
| 1 | 931 | α-Pinene | 0.17 |
| 2 | 957 | Camphene | 0.55 |
| 3 | 981 | β-Pinene | 0.27 |
| 4 | 1030 | (D)-Limonene | 0.56 |
| 5 | 1032 | 1,8-Cineol | 0.16 |
| 6 | 1094 | Linalool | 1.07 |
| 7 | 1146 | Camphor | 2.42 |
| 8 | 1179 | Terpinen-4-ol | 1.21 |
| 9 | 1191 | α-Terpineol | 0.66 |
| 10 | 1198 | Estragole | 0.89 |
| 11 | 1287 | Bornyl acetate | 0.13 |
| 12 | 1374 | Copaene | 1.08 |
| 13 | 1400 | α-Funebrene | 0.23 |
| 14 | 1406 | α-Gurjunene | 0.69 |
| 15 | 1409 | α-Cedrene | 3.09 |
| 16 | 1416 | α-Bulnesene | 0.32 |
| 17 | 1403 | Methyleugenol | 8.59 |
| 18 | 1422 | β-Cedrene | 1.52 |
| 19 | 1420 | β-Caryophyllene | 0.21 |
| 20 | 1454 | α-Caryophyllene | 0.19 |
| 21 | 1500 | (E)-Methylisoeugenol | 14.01 |
| 22 | 1458 | Germacrene D | 2.12 |
| 23 | 1504 | Cuparene | 0.73 |
| 24 | 1537 | α-Cadinene | 1.35 |
| 25 | 1557 | β-Calacorene | 0.51 |
| 26 | 1561 | Germacrene B | 0.26 |
| 27 | 1631 | β-Asarone | 3.51 |
| 28 | 1654 | α-Cadinol | 0.27 |
| 29 | 1678 | α-Asarone | 50.09 |
| 30 | 1685 | Acorenone | 0.23 |
| 31 | 1686 | α-Bisabolol | 0.13 |
| 32 | 1775 | Acorone | 0.11 |
| Total | 97.52 | ||
| Monoterpenoids | 7.20 | ||
| Sesquiterpenoids | 13.04 | ||
| Others | 77.28 |
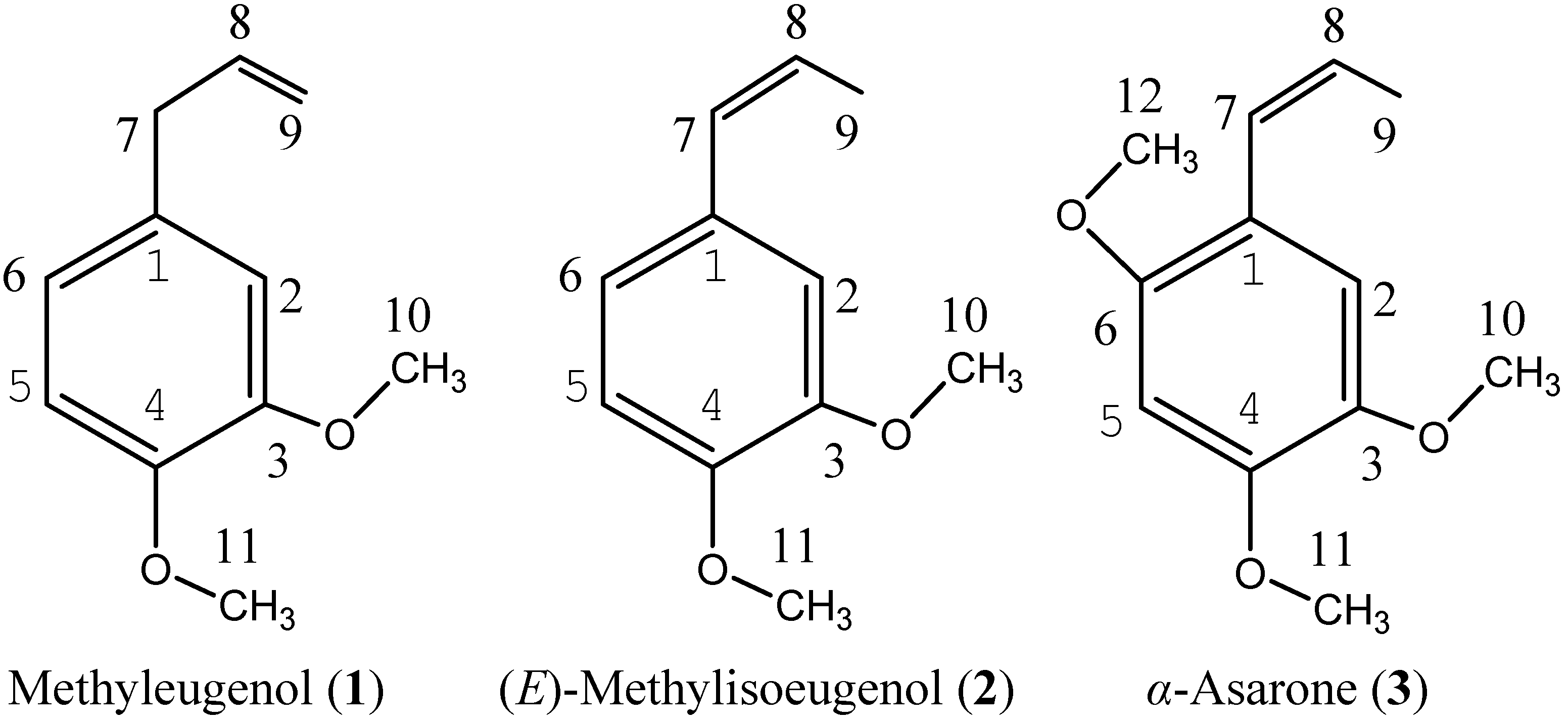
2.2. Insecticidal Activities
| Treatment | LD50/LC50 | 95% FL * | Slope ± SE | Chi square (χ2) | |
|---|---|---|---|---|---|
| Contact Toxicity (μg/cm2) | A. calamus | 100.21 | 93.19–108.23 | 7.29 ± 0.89 | 23.52 |
| α-Asarone | 125.73 | 114.45–136.89 | 11.03 ± 1.25 | 15.12 | |
| Methyleugenol | 103.22 | 94.87–112.19 | 9.47 ± 1.02 | 13.16 | |
| (E)-Methylisoeugenol | 55.32 | 50.89–59.63 | 5.34 ± 0.62 | 15.87 | |
| Pyrethrum extract | 18.99 | 17.56–20.06 | 7.64 ± 1.05 | - | |
| Fumigant (μg/L air) | A. calamus | 392.13 | 365.76–430.54 | 6.81 ± 0.70 | 10.92 |
| α-Asarone | >2060.00 | - | - | - | |
| Methyleugenol | 92.21 | 83.29–100.78 | 3.50 ± 0.31 | 13.16 | |
| (E)-Methylisoeugenol | 143.43 | 131.19–157.65 | 4.13 ± 0.40 | 9.81 | |
| Dichlorvos | 1.35 | 1.25–1.47 | 6.87 ± 0.77 | - |
3. Experimental
3.1. Plant Material and Essential Oil Extraction
3.2. Insects
3.3. Gas Chromatography-Mass Spectrometry
3.4. Purification and Characterization of Three Constituent Compounds
3.5. Isolated Constituent Compounds
3.6. Contact Toxicity with Treated Filter Paper
3.7. Fumigant Toxicity
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Nayak, M.K.; Daglish, G.J.; Byrne, V.S. Effectiveness of spinosad as a grain protectant against resistant beetle and psocid pests of stored grain in Australia. J. Stored Prod. Res. 2005, 41, 455–467. [Google Scholar] [CrossRef]
- Turner, B.D. Psocids as a nuisance problem in the UK. Pestic. Outlook 1998, 9, 27–30. [Google Scholar]
- Pascual-Villalobos, M.J.; Baz, A.; Del Estal, P. Occurrence of psocids and natural predators on organic rice in Calasparra (Murcia, Spain). J. Stored Prod. Res. 2005, 41, 231–235. [Google Scholar] [CrossRef]
- Zettler, J.L.; Arthur, F.H. Chemical control of stored product insects with fumigants and residual treatments. Crop Prot. 2000, 19, 577–582. [Google Scholar] [CrossRef]
- Phillips, T.W.; Throne, J.E. Biorational approaches to managing stored-product pests. Ann.Rev.Entomol. 2010, 55, 375–397. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Ann. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Rajendran, S.; Srianjini, V. Plant products as fumigants for stored-product insects control. J. Stored Prod. Res. 2008, 44, 126–135. [Google Scholar] [CrossRef]
- Chu, S.S.; Liu, Q.R.; Liu, Z.L. Insecticidal activity and chemical composition of the essential oil of Artemisia vestita from China. Biochem. Syst. Ecol. 2010, 38, 489–492. [Google Scholar] [CrossRef]
- Li, W.Q.; Jiang, C.H.; Chu, S.S.; Zuo, M.X.; Liu, Z.L. Chemical composition and toxicity against Sitophilus zeamais and Tribolium castaneum of the essential oil of Murraya exotica aerial parts. Molecules 2010, 15, 5831–5839. [Google Scholar] [CrossRef]
- Fang, R.; Jiang, C.H.; Wang, X.Y.; Zhang, H.M.; Liu, Z.L.; Zhou, L.; Du, S.S.; Deng, Z.W. Insecticidal activity of essential oil of Carum carvi fruits from China and its main components against two grain storage insects. Molecules 2010, 15, 9391–9402. [Google Scholar] [CrossRef]
- Chu, S.S.; Hu, J.F.; Liu, Z.L. Composition of essential oil of Chinese Chenopodium ambrosioides and insecticidal activities to maize weevil, Sitophilus zeamais. Pest Manag. Sci. 2011, 67, 714–718. [Google Scholar] [CrossRef]
- Zhang, J.S.; Zhao, N.N.; Liu, Q.Z.; Liu, Z.L.; Du, S.S.; Zhou, L.; Deng, Z.W. Repellent constituents of essential oil of Cymbopogon distans aerial parts against two stored-product insects. J. Agric. Food Chem. 2011, 59, 9910–9915. [Google Scholar]
- Chu, S.S.; Jiang, G.H.; Liu, Z.L. Insecticidal compounds from the essential oil of Chinese medicinal herb, Atractylodes chinensis. Pest Manag. Sci. 2011, 67, 1253–1257. [Google Scholar] [CrossRef]
- Yang, K.; Zhou, Y.X.; Wang, C.F.; Du, S.S.; Deng, Z.W.; Liu, Q.Z.; Liu, Z.L. Toxicity of Rhododendron anthopogonoides essential oil and its constituent compounds towards Sitophilus zeamais. Molecules 2011, 16, 7320–7330. [Google Scholar] [CrossRef]
- Zhao, N.N.; Zhou, L.; Liu, Z.L.; Du, S.S.; Deng, Z.W. Evaluation of toxicities of some common spices essential oils from China against Liposcelis bostrychophila. Food Control 2012, 26, 486–490. [Google Scholar] [CrossRef]
- Liu, Z.L.; Zhao, N.N.; Liu, C.M.; Zhou, L.; Du, S.S. Identification of insecticidal constituents of the essential oil of Curcuma wenyujin rhizomes against Liposcelis bostrychophila Badonnel. Molecules 2012, 17, 12049–12060. [Google Scholar] [CrossRef]
- Liang, Y.; Li, J.L.; Xu, S.; Zhao, N.N.; Zhou, L.; Cheng, J.; Liu, Z.L. Evaluation of repellency of some Chinese medicinal herbs essential oils against Liposcelis bostrychophila (Psocoptera: Liposcelidae) and Tribolium castaneum (Coleoptera: Tenebrionidae). J. Econ. Entomol. 2013, 106, 513–519. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhao, J.L.; Liu, H.; Zhou, L.; Liu, Z.L.; Han, J.G.; Zhu, Y.; Yang, F.Y. Chemical analysis and biological activity of the essential oils of two Valerianaceous species from China: Nardostachys chinensis and Valeriana officinalis. Molecules 2010, 15, 6411–6422. [Google Scholar] [CrossRef]
- Liu, Z.L.; He, Q.; Chu, S.S.; Wang, C.F.; Du, S.S.; Deng, Z.W. Essential oil composition and larvicidal activity of Saussurea lappa roots against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2012, 110, 2125–2130. [Google Scholar] [CrossRef]
- Liu, Z.L.; Liu, Q.Z.; Du, S.S.; Deng, Z.W. Mosquito larvicidal activity of alkaloids and limonoids derived from Evodia rutaecarpa unripe fruits against Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2012, 111, 991–996. [Google Scholar] [CrossRef]
- Liu, X.C.; Dong, H.W.; Zhou, L.; Du, S.S.; Liu, Z.L. Essential oil composition and larvicidal activity of Toddalia asiatica roots against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2013, 112, 1197–1203. [Google Scholar] [CrossRef]
- Araujo, M.J.; Camara, C.A.; Born, F.S.; Moraes, M.M.; Badji, C.A. Acaricidal activity and repellency of essential oil from Piper aduncum and its components against Tetranychus urticae. Exp. Appl. Acarol. 2012, 57, 139–155. [Google Scholar] [CrossRef]
- Li, H.Q.; Bai, C.Q.; Chu, S.S.; Zhou, L.; Du, S.S.; Liu, Z.L.; Liu, Q.Z. Chemical composition and toxicities of the essential oil derived from Kadsura heteroclita stems against Sitophilus zeamais and Meloidogyne incognita. J. Med. Plants Res. 2011, 5, 4943–4948. [Google Scholar]
- Bai, C.Q.; Liu, Z.L.; Liu, Q.Z. Nematicidal constituents from the essential oil of Chenopodium ambrosioides aerial parts. Eur.-J. Chem. 2011, 8, 143–148. [Google Scholar]
- Liu, Q.Z.; Li, H.Q.; Liu, Z.L. Nematicidal constituents from the ethanol extract of Evodia rutaecarpa Hort unripe fruits. J. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Flora of China. Available online: Available online: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200027130 (accessed on 4 May 2013).
- Dong, W.W.; Yang, D.J.; Lu, R.H. Chemical constituents from the rhizome of Acorus calamus L. Planta Med. 2010, 76, 454–457. [Google Scholar] [CrossRef]
- Keller, K.; Stahl, E. Composition of the essential oil from β-asarone free calamus. Planta Med. 1983, 47, 71–74. [Google Scholar] [CrossRef]
- Huang, Y.Z.; He, Z.Y.; Gao, Y.H.; Wu, J.L. Analysis of the components of the rhizome volatile oils from Chinese Acorus plants and rational use of the resources. Chin. J. Chromatogr. 1993, 110, 267–270. [Google Scholar]
- Ozcan, M.; Akgul, A.; Chalchat, J.C. Volatile constituents of the essential oil of Acorus calamus L. grown in Konya province (Turkey). J. Essent. OilRes. 2002, 14, 366–368. [Google Scholar] [CrossRef]
- Venskutonis, P.R.; Dagilyte, A. Composition of essential oil of sweet flag (Acorus calamus L.) leaves at different growing phases. J. Essent. Oil Res. 2003, 15, 313–318. [Google Scholar] [CrossRef]
- Marongiu, B.; Piras, A.; Porcedda, S.; Scorciapino, A. Chemical composition of the essential oil and supercritical CO2 extract of Commiphora myrrha (Nees) Engl. and of Acorus calamus L. J. Agric. Food Chem. 2005, 53, 7939–7943. [Google Scholar] [CrossRef]
- Garneau, F.X.; Collin, G.; Gagnon, H.; Belanger, A.; Lavoie, S.; Savard, N.; Pichette, A. Aromas from Quebec. I. Composition of the essential oil of the rhizomes of Acorus calamus L. J. Essent. Oil Res. 2008, 20, 250–254. [Google Scholar] [CrossRef]
- Gong, X.L.; Dian, L.H.; Zhang, L.J. Study on chemical constituents of volatile oil in rhizome and root of Acorus calamus L. (Chinese). Chin. Pharm. 2007, 18, 176–178. [Google Scholar]
- Zhang, L.S.; Dong, G.D.; Liu, G.M. Study on chemical constituents of essential oil from Acorus calamus from two different habitats (Chinese). Chin. Pharm. 2010, 21, 2153–2155. [Google Scholar]
- Lin, C.L.; Lin, G.Y.; Cai, J.Z. Study on the chemical constituents of the volatile oils from the Acorus calamus growing in Zhejiang Province. Chin. Pharm. 2012, 23, 640–641. [Google Scholar]
- Ramos-Ocampo, V.E.; Hsia, M.T.S. Toxicity and chemosterilant activity of calamus oil and asarone analogs to the kelp fly, Coelopa frigida (F.). Philipp. Entomol. 1986, 6, 485–494. [Google Scholar]
- Tare, V. Bioactivity of some medicinal plants against chosen insect pests/vectors. J. Med. Aromatic Plant Sci. 2001, 22, 120–124. [Google Scholar]
- Schmidt, G.H.; Streloke, M. Effect of Acorus calamus (L.) (Araceae) oil and its main compound β-asarone on Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae). J. Stored Prod. Res. 1994, 30, 227–235. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, O.P.; Saxena, B.P. Effect of sweet flag rhizome oil (Acorus calamus) on hemogram and ultrastructure of hemocytes of the tobacco armyworm, Spodoptera litura (Lepidoptera: Noctuidae). Micron 2008, 39, 544–551. [Google Scholar] [CrossRef]
- Rahman, M.M.; Schmidt, G.H. Effect of Acorus calamus (L.) (Araceae) essential oil vapours from various origins on Callosobruchus phaseoli (Gyllenhal) (Coleoptera: Bruchidae). J. Stored Prod. Res. 1999, 35, 285–295. [Google Scholar] [CrossRef]
- Yao, Y.J.; Cai, W.L.; Yang, C.J.; Xue, D.; Huang, Y.Z. Isolation and characterization of insecticidal activity of (Z)-asarone from Acorus calamus L. Insect Sci. 2008, 15, 229–236. [Google Scholar] [CrossRef]
- Hu, Y.J.; Yang, W.Q.; Cai, W.L.; Yang, C.J.; Hua, H.X. Comparison of biological activity of Acorus calamus with different extract methods against Blattella germanica. Chin. Agric. Sci. Bull. 2009, 25, 213–217. [Google Scholar]
- Fujita, S.I.; Enornata, Y.; Suernitsu, R.; Fujita, Y. Miscellaneous contributions to the essential oils of the plants from various territories XXVII. On the components of the essential oils of Acorus calamus Linn. var. Angustatus Bess. Yakagaka Zasshi 1971, 91, 571–574. [Google Scholar]
- Li, M.X.; Jiang, Z.R. The volatile constituents of the essential oil and their distribution in Acorus calamus L. Chin. Trad. Herb. Drug. 1993, 24, 459–461. [Google Scholar]
- Rost, L.C.M.; Bos, R. Biosystematic investigations with Acorus L. Planta Med. 1979, 36, 350–361. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, S.H.; Lee, H.C.; Yap, Y.L. Insecticidal properties of eugenol, isoeugenol and methyleugenol and their effects on nutrition of Sitophilus zeamais Motsch. (Coleoptera: Curculionidae) and Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2002, 38, 403–412. [Google Scholar] [CrossRef]
- Perumalsamy, H.; Chang, K.S.; Park, C.; Ahn, Y.J. Larvicidal activity of Asarum heterotropoides root constituents against insecticide-susceptible and -resistant Culex pipiens pallens and Aedes aegypti and Ochlerotatus togoi. J. Agric. Food Chem. 2010, 58, 10001–10006. [Google Scholar] [CrossRef]
- Ngoh, S.P.; Choo, L.E.W.; Pang, F.Y.; Huang, Y.; Kini, M.R.; Ho, S.H. Insecticidal and repellent properties of nine volatile constituents of essential oils against the American cockroach, Periplaneta americana (L.). Pesticide Sci. 1998, 54, 261–268. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, H.K.; Choi, D.H.; Ahn, Y.J. Acaricidal activity of clove bud oil compounds against Tyrophagus putrescentiae (Acari: Acaridae). Appl. Entomol. Zool. 2003, 38, 261–266. [Google Scholar]
- Kim, E.H.; Kim, H.K.; Ahn, Y.J. Acaricidal activity of clove bud oil compounds against Dermatophagoides farinae and Dermatophagoides pteronyssinus (Acari: Pyroglyphidae). J. Agric. Food Chem. 2003, 51, 885–889. [Google Scholar] [CrossRef]
- Sung, B.K.; Lee, H.S. Chemical composition and acaricidal activities of constituents derived from Eugenia caryophyllata leaf oils. Food Sci. Biotechnol. 2005, 14, 73–76. [Google Scholar]
- Park, C.; Kim, S.I.; Ahn, Y.J. Insecticidal activity of asarones identified in Acorus gramineus rhizome against three coleopteran stored-product insects. J. Stored Prod. Res. 2003, 39, 333–342. [Google Scholar] [CrossRef]
- Lee, H.K.; Park, C.; Ahn, Y.J. Insecticidal activities of asarones identified in Acorus gramineus rhizome against Nilaparvata lugens (Homoptera: Delphacidae) and Plutella xylostella (Lepidoptera: Yponomeutoidae). Appl. Entomol. Zool. 2002, 37, 459–464. [Google Scholar] [CrossRef]
- Saxena, B.P.; Koul, O.; Tikku, K.; Atal, C.K. A new insect chemosterilant isolated from Acorus calamus L. Nature 1977, 270, 512–513. [Google Scholar] [CrossRef]
- Koul, O.; Smirle, M.J.; Isman, M.B. Asarones from Acorus calamus L. ooil. Their effect on feeding behavior and dietary utilization in Peridroma saucia. J. Chem. Ecol. 1990, 16, 1911–1920. [Google Scholar] [CrossRef]
- Perrett, S.; Whitfield, P.J. Anthelmintic and pesticidal activity of Acorus gramineus (Araceae) is associated with phenylpropanoid asarones. Phytother. Res. 1995, 9, 405–409. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured: Carol Stream, IL, USA, 2007. [Google Scholar]
- Miyazawa, M.; Hisama, M. Suppression of chemical mutagen-induced SOS response by alkylphenols from clove (Syzygium aromaticum) in the Salmonella typhimurium TA1535/pSK1002 umu test. J. Agric. Food Chem. 2001, 49, 4019–4025. [Google Scholar] [CrossRef]
- Zuo, H.L.; Yang, F.Q.; Zhang, X.M.; Xia, Z.N. Separation of cis- and trans-asarone from Acorus tatarinowii by preparative gas chromatography. J. Anal. Methods Chem. 2012, 2012. [Google Scholar] [CrossRef]
- Sakuma, M. Probit analysis of preference data. Appl. Entomol. Zool. 1998, 33, 339–347. [Google Scholar]
- Sample Availability: Samples of the crude extracts and pure compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, X.C.; Zhou, L.G.; Liu, Z.L.; Du, S.S. Identification of Insecticidal Constituents of the Essential Oil of Acorus calamus Rhizomes against Liposcelis bostrychophila Badonnel. Molecules 2013, 18, 5684-5696. https://doi.org/10.3390/molecules18055684
Liu XC, Zhou LG, Liu ZL, Du SS. Identification of Insecticidal Constituents of the Essential Oil of Acorus calamus Rhizomes against Liposcelis bostrychophila Badonnel. Molecules. 2013; 18(5):5684-5696. https://doi.org/10.3390/molecules18055684
Chicago/Turabian StyleLiu, Xin Chao, Li Gang Zhou, Zhi Long Liu, and Shu Shan Du. 2013. "Identification of Insecticidal Constituents of the Essential Oil of Acorus calamus Rhizomes against Liposcelis bostrychophila Badonnel" Molecules 18, no. 5: 5684-5696. https://doi.org/10.3390/molecules18055684
APA StyleLiu, X. C., Zhou, L. G., Liu, Z. L., & Du, S. S. (2013). Identification of Insecticidal Constituents of the Essential Oil of Acorus calamus Rhizomes against Liposcelis bostrychophila Badonnel. Molecules, 18(5), 5684-5696. https://doi.org/10.3390/molecules18055684






