The Development, Physicochemical Characterisation and in Vitro Drug Release Studies of Pectinate Gel Beads Containing Thai Mango Seed Kernel Extract
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of MSKE-Loaded Pectinate Beads
2.2. Particle Size and Shape
| Formulation parameters | Size (mm ± SD) n = 30 | Shape (ER ± SD) n = 30 | EE j (%) | ||
|---|---|---|---|---|---|
| Constants | Variables | Values | |||
| MSKE (4%w/v) CaCl2 (6% w/v) | Pectin:MSKE (w/w) | 1.00:1.00 | 1.13 ± 0.05 a | 1.10 ± 0.01 | 42.52 ± 1.78 k |
| 1.25:1.00 | 1.36 ± 0.02 b | 1.15 ± 0.01 | 49.80 ± 2.16 l | ||
| 1.50:1.00 | 1.50 ± 0.01 c | 1.13 ± 0.01 | 54.43 ± 0.43 m | ||
| 1.75:1.00 | 1.63 ± 0.10 d | 1.13 ± 0.05 | 58.36 ± 0.38 n | ||
| CaCl2 (6% w/v) | Pectin:MSKE (w/w) | 4:4 | 1.13 ± 0.05 A | 1.10 ± 0.01 | 42.52 ± 1.78 K |
| 5:5 | 1.40 ± 0.04 B | 1.12 ± 0.03 | 53.21 ± 2.84 L | ||
| 6:6 | 1.43 ± 0.02 C | 1.15 ± 0.02 | 61.42 ± 3.01 M | ||
| 7:7 | 1.54 ± 0.10 D | 1.11 ± 0.03 | 69.65 ± 0.71 N | ||
| MSKE (7% w/v) Pectin (7%w/v) | CaCl2(%w/v) | 6 | 1.54 ± 0.10 e | 1.11 ± 0.03 | 69.65 ± 0.71 r |
| 7 | 1.60 ± 0.05 e | 1.09 ± 0.01 | 67.69 ± 1.44 r | ||
| 8 | 1.62 ± 0.10 e | 1.08 ± 0.03 | 67.17 ± 2.43 r | ||
| 9 | 1.63 ± 0.01 e | 1.09 ± 0.02 | 67.21 ± 0.37 r | ||
| 10 | 1.70 ± 0.10 e | 1.07 ± 0.02 | 65.21 ± 1.96 r | ||
| MSKE (7% w/v) Pectin (7% w/v) | Zn(CH3COO)2 (%w/v) | 1 | 1.86 ± 0.02 E | 1.00 ± 0.01 | 100.22 ± 0.53 R |
| 2 | 1.87 ± 0.01 E | 1.01 ± 0.01 | 100.12 ± 1.59 R | ||
| 4 | 1.86 ± 0.05 E | 1.00 ± 0.00 | 97.64 ± 5.6 2 R | ||
| 6 | 1.88 ± 0.01 E | 1.01 ± 0.01 | 104.48 ± 8.15 R | ||
2.3. Scanning Electron Microscopy (SEM)
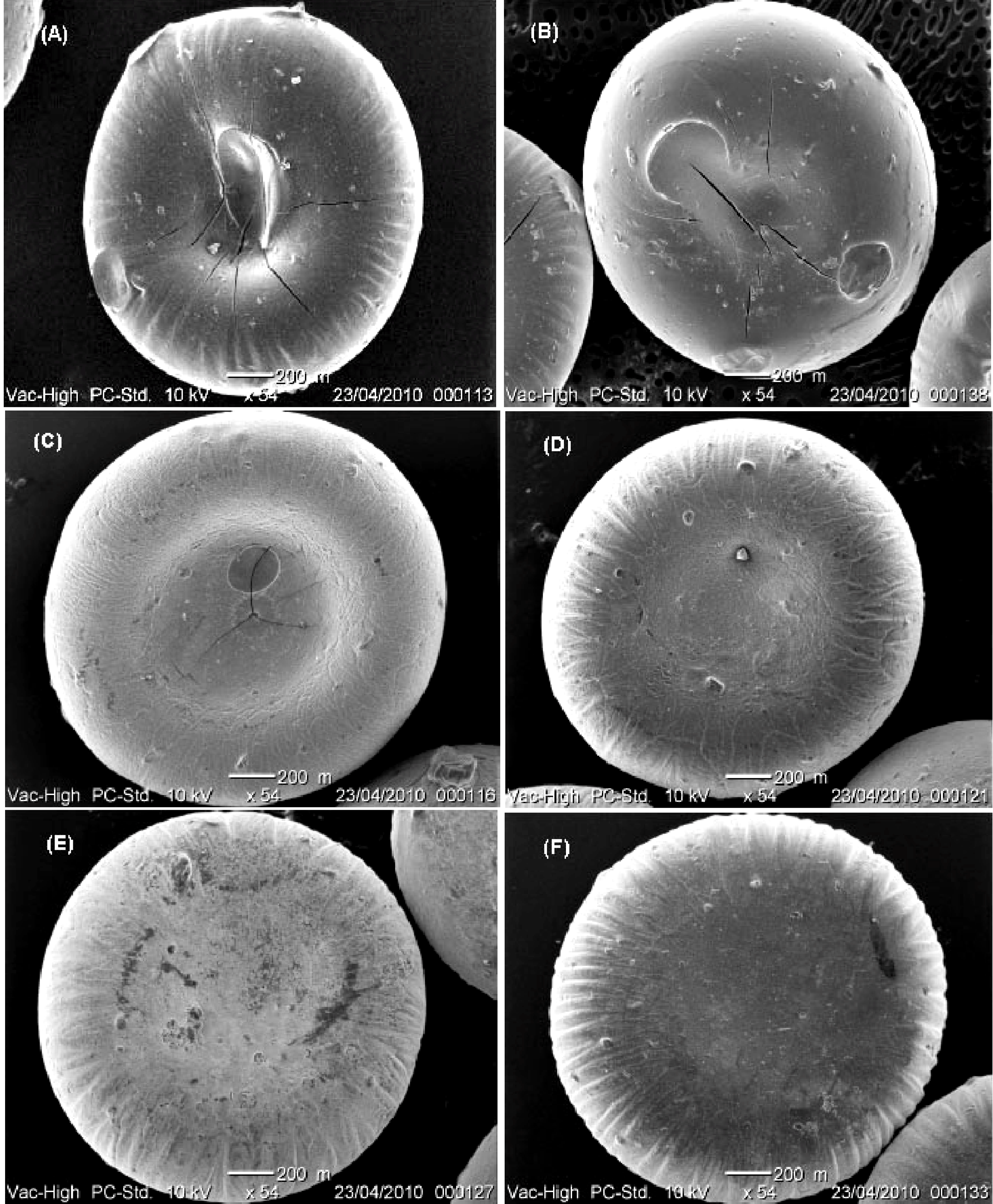
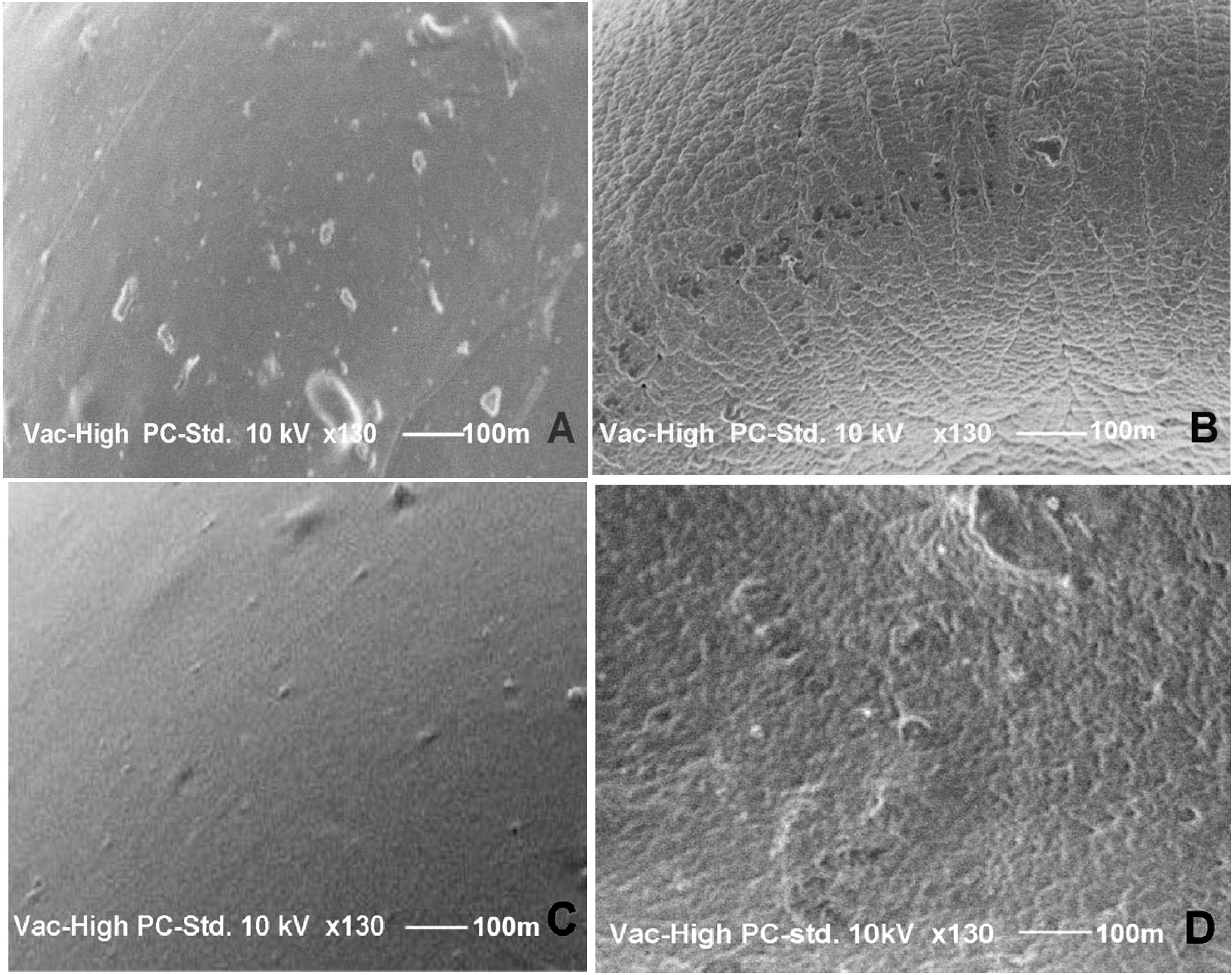
2.4. Entrapment Efficiency (EE)
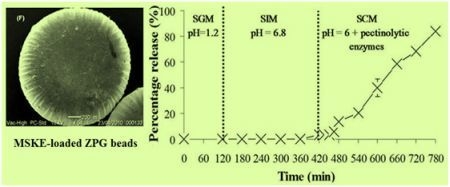
2.5. In Vitro Release Studies of MSKE-Loaded Pectinate Beads
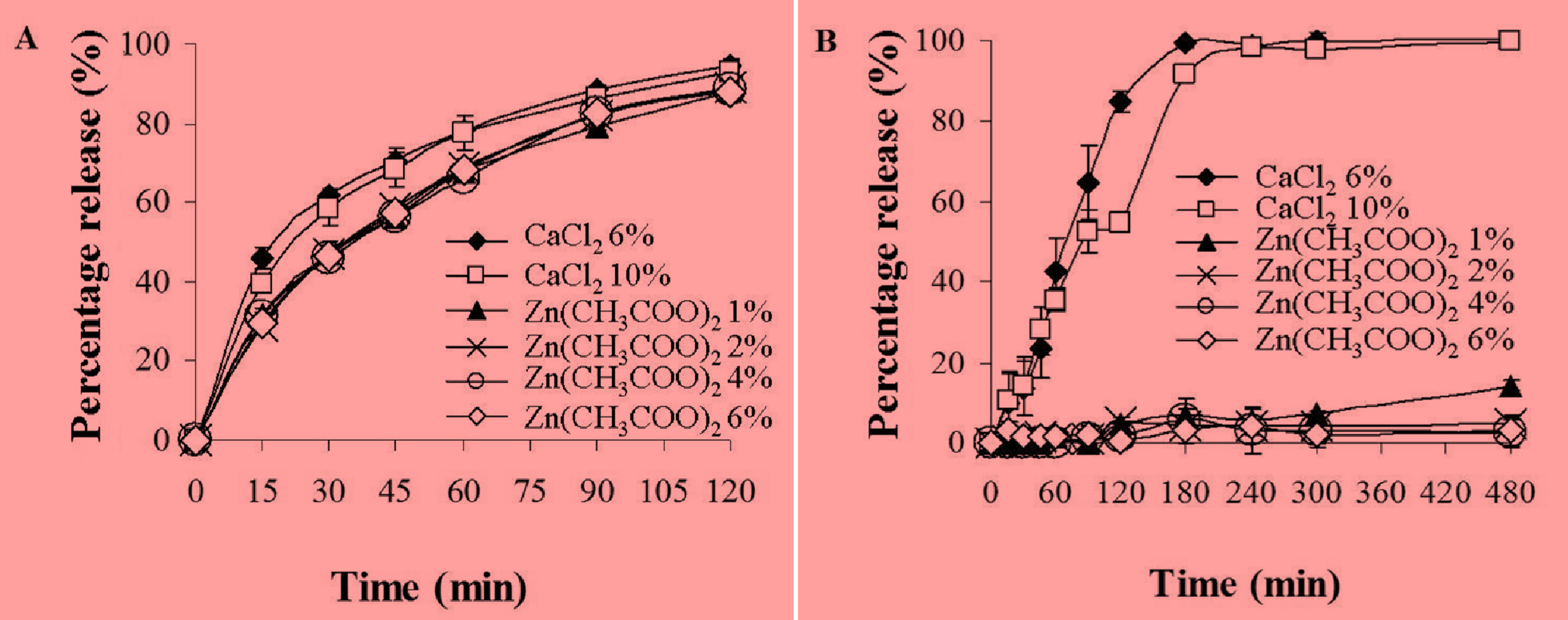
| Percentage release of MSKE with different % Zn(CH3COO)2 | ||||
|---|---|---|---|---|
| 1% | 2% | 4% | 6% | |
| SCM without enzymes | 101.14 ± 2.20 | 4.00 ± 0.56 | 3.04 ± 0.65 | 3.16 ± 0.21 |
| SCM with enzymes; 650 U/mL | NT | 25.55 ± 0.78 | 3.15 ± 1.19 | 3.13 ± 0.47 |
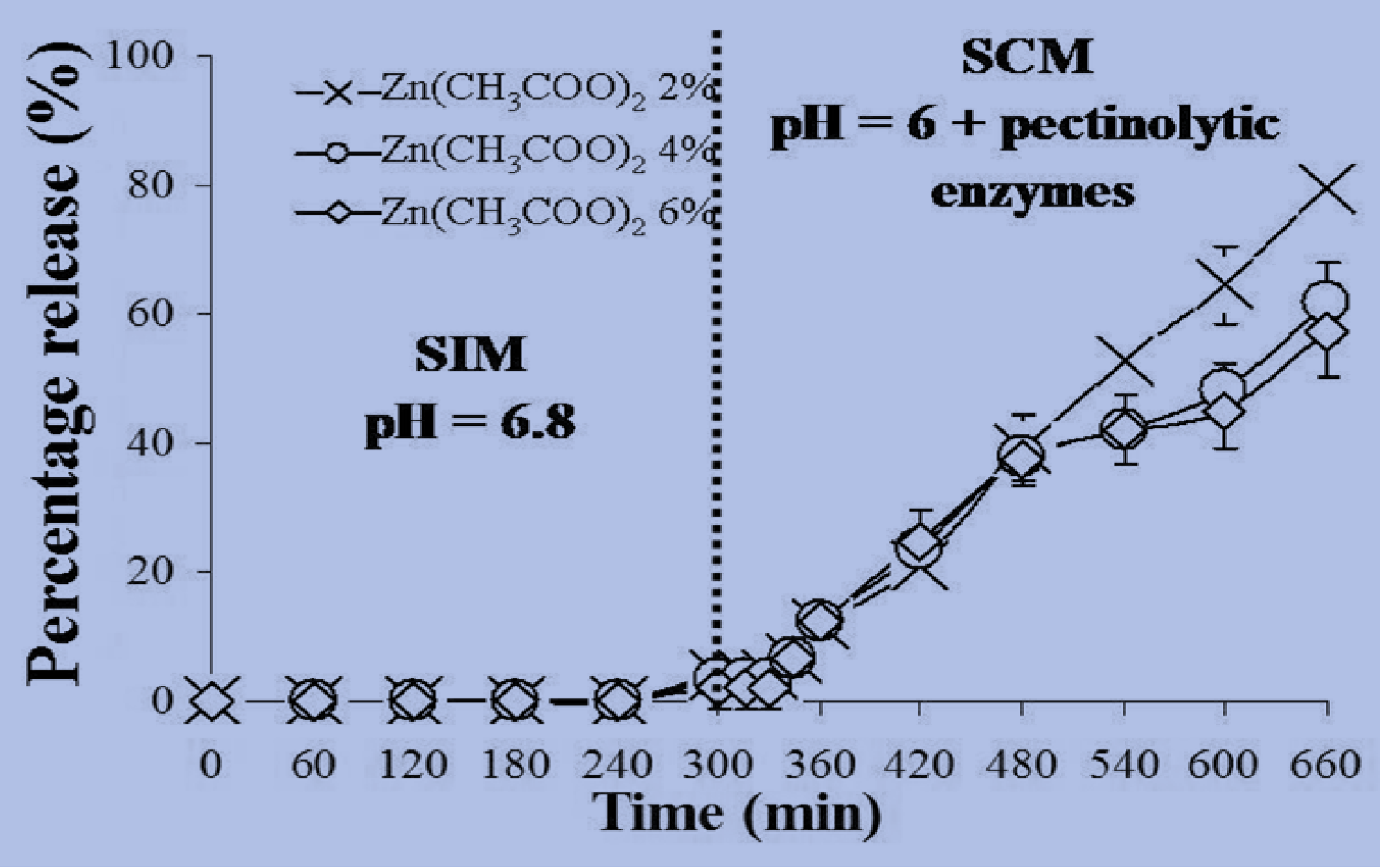
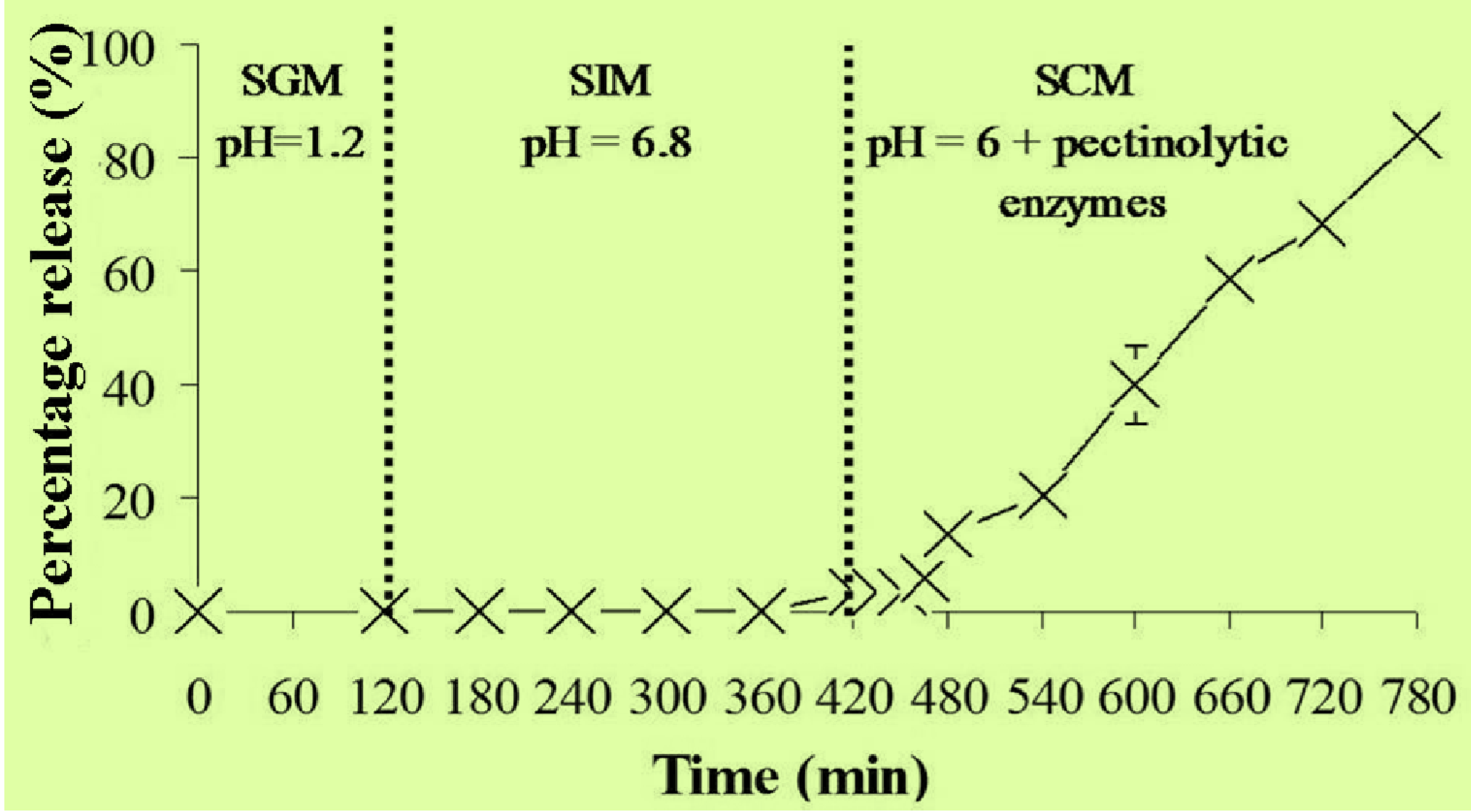
2.6. Stability Study
| Temperature | % EE of MSKE with different % Zn(CH3COO)2 | ||
|---|---|---|---|
| 2% | 4% | 6% | |
| 4 °C | 98.78 ± 1.89 | 99.55 ± 1.73 | 98.57 ± 0.79 |
| 25 °C | 99.33 ± 0.33 | 99.65 ± 0.88 | 100.85 ± 1.64 |
| 45 °C | 99.46 ± 1.19 | 99.95 ± 2.36 | 99.53 ± 0.98 |
3. Experimental
3.1. Chemicals
3.2. Preparation of MSKE
3.3. Analysis of PGG Content in MSKE
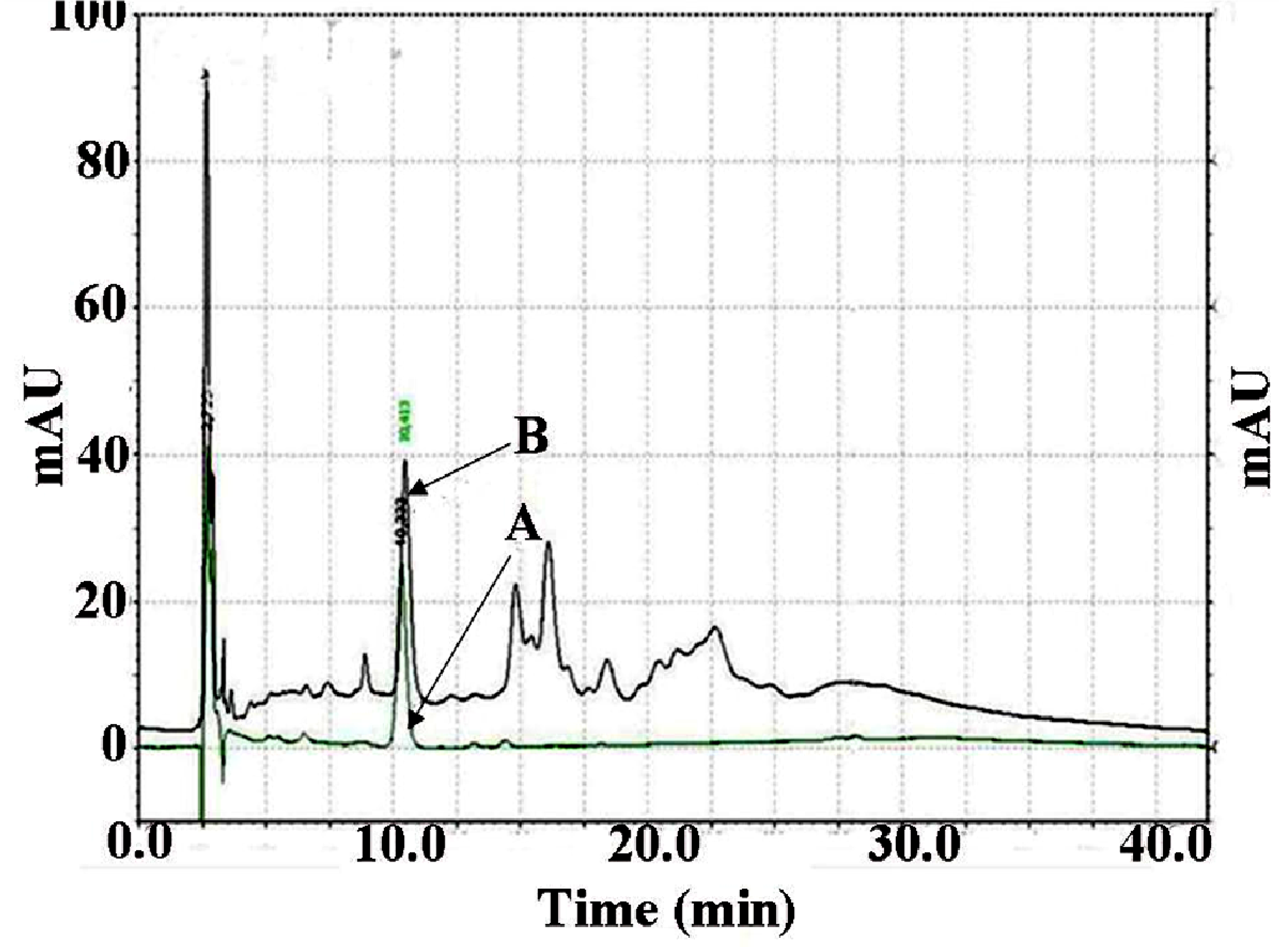
3.4. Preparation of MSKE-Loaded Pectinate Gel Beads
3.5. Characterization of Pectin Beads
3.5.1. Particle Size and Shape
3.5.2. SEM
3.5.3. Determination of EE
3.5.4. In Vitro Release Studies of MSKE-loaded Pectinate Beads
3.6. Stability Study
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Haslam, E. Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef]
- Sharma, L.D.; Bahga, H.S.; Srivastava, P.S. In vitro anthelmintic screening of indigenous medicinal plants against Haemonchus contortus (Rudolphi, 1803) Cobbold, 1898 of sheep and goats. Indian J. Anim. Res. 1971, 5, 33–38. [Google Scholar]
- Singh, Y.N. Traditional medicine in Fiji. Some herbal folk cures used by Fiji Indians. J. Ethnopharmacol. 1986, 15, 57–88. [Google Scholar]
- Coe, F.G.; Anderson, G.J. Screening of medicinal plants used by the Garifuna of eastern Nicaragua for bioactive compounds. J. Ethnopharmacol. 1996, 53, 29–50. [Google Scholar]
- John, D. One hundred useful raw drugs of the Kani Tribes of Trivandrum Forest Division, Kerala, India. Pharmaceut. Biol. 1984, 22, 17–39. [Google Scholar] [CrossRef]
- Das, P.C.; Das, A.; Mandal, S.; Islam, C.N.; Dutta, M.K.; Patra, B.B.; Sikdar, S.; Chakrabarthy, P.K., 1989. Antiinflammatory and antimicrobial activities of the seed kernel of Mangifera indica. Fitoterapia 1989, 60, 235–240. [Google Scholar]
- Soong, Y.Y.; Barlow, P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004, 88, 411–417. [Google Scholar]
- Nithitanakool, S.; Pithayanukul, P.; Bavovada, R. Antioxidant and hepatoprotective activities of Thai mango seed kernel extract. Planta Med. 2009, 75, 1118–1123. [Google Scholar]
- Nithitanakool, S.; Pithayanukul, P.; Bavovada, R.; Saparpakorn, P. Molecular docking studies and anti-tyrosinase activity of Thai mango seed kernel extract. Molecules 2009, 14, 257–265. [Google Scholar]
- Jiamboonsri, P.; Pithayanukul, P.; Bavovada, R.; Chomnawang, M.T. The inhibitory potential of Thai mango seed kernel extract against Methicillin-Resistant Staphylococcus Aureus. Molecules 2011, 16, 6255–6270. [Google Scholar]
- Zhang, J.; Li, L.; Kim, S.H.; Hagerman, A.E.; Lü, J. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm. Res. 2009, 26, 2066–2080. [Google Scholar]
- Sinha, V.R.; Kumria, R. Polysaccharides in colon-specific drug delivery. Int. J. Pharm. 2001, 224, 19–38. [Google Scholar]
- Mathiowitz, E. Oral drug delivery, small intestine and colon. In Encyclopedia of Controlled Drug Delivery 2; Mathiowitz, E., Ed.; John Wiley & Sons, Inc.: New Jersey, NJ, USA, 1999; pp. 717–728. [Google Scholar]
- Cummings, J.H.; Southgate, D.A.T.; Branch, W.J.; Wiggins, H.S.; Houston, H.; Jenkins, D.J.A.; Jivraj, T.; Hill, M.J. The digestion of pectin in the human gut and its effect on calcium absorption and large bowel function. Br. J. Nutr. 1979, 41, 477–485. [Google Scholar]
- Liu, L.; Fishman, M.L.; Kost, J.; Hicks, K.B. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 2003, 24, 3333–3343. [Google Scholar]
- Aydin, Z.; Akbuğa, J. Preparation and evaluation of pectin beads. Int. J. Pharm. 1996, 137, 133–136. [Google Scholar]
- Bourgeois, S.; Gernet, M.; Pradeau, D.; Andremont, A.; Fattal, E. Evaluation of critical formulation parameters influencing the bioactivity of β-lactamases entrapped in pectin beads. Int. J. Pharm. 2006, 324, 2–9. [Google Scholar]
- El-Gibaly, I. Oral delayed-release system based on Zn-pectinate gel (ZPG) microparticles as an alternative carrier to calcium pectinate beads for colonic drug delivery. Int. J. Pharm. 2002, 232, 199–211. [Google Scholar] [CrossRef]
- Atyabi, F.; Majzoob, S.; Iman, M.; Salehi, M.; Dorkoosh, F. In vitro evaluation and modification of pectinate gel beads containing trimethyl chitosan, as a multi-particulate system for delivery of water-soluble macromolecules to colon. Carbohydr. Polym. 2005, 61, 39–51. [Google Scholar] [CrossRef]
- Chambin, O.; Dupuis, G.; Champion, D.; Voilley, A.; Pourcelot, Y. Colon-specific drug delivery: Influence of solution reticulation properties upon pectin beads performance. Int. J. Pharm. 2006, 321, 86–93. [Google Scholar]
- Bajpai, S.K.; Sharma, S. Investigation of swelling/degradation behavior of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Kennedy, R.A. Swelling and diffusion studies of calcium polysaccharide gels intended for film coating. Int. J. Pharm. 2008, 358, 205–213. [Google Scholar] [CrossRef]
- Dronnet, V.M.; Renard, C.M.G.C.; Axelos, M.A.V.; Thibault, J.-F. Characterisation and selectivity of divalent metal ions binding by citrus and sugar-beet pectins. Carbohydrate Polym. 1996, 30, 253–263. [Google Scholar] [CrossRef]
- Semdé, R.; Amighi, K.; Devleeschouwer, M.J.; Moës, A.J. Effect of pectinolytic enzymes on the theophylline release from pellets coated with water insoluble polymers containing pectin HM or calcium pectinate. Int. J. Pharm. 2000, 197, 169–179. [Google Scholar]
- Wong, T.W.; Nurjaya, S. Drug release property of chitosan-pectinate beads and its changes under the influence of microwave. Eur. J. Pharm. Biopharm. 2008, 69, 176–188. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nithitanakool, S.; Pithayanukul, P.; Bourgeois, S.; Fessi, H.; Bavovada, R. The Development, Physicochemical Characterisation and in Vitro Drug Release Studies of Pectinate Gel Beads Containing Thai Mango Seed Kernel Extract. Molecules 2013, 18, 6504-6520. https://doi.org/10.3390/molecules18066504
Nithitanakool S, Pithayanukul P, Bourgeois S, Fessi H, Bavovada R. The Development, Physicochemical Characterisation and in Vitro Drug Release Studies of Pectinate Gel Beads Containing Thai Mango Seed Kernel Extract. Molecules. 2013; 18(6):6504-6520. https://doi.org/10.3390/molecules18066504
Chicago/Turabian StyleNithitanakool, Saruth, Pimolpan Pithayanukul, Sandrine Bourgeois, Hatem Fessi, and Rapepol Bavovada. 2013. "The Development, Physicochemical Characterisation and in Vitro Drug Release Studies of Pectinate Gel Beads Containing Thai Mango Seed Kernel Extract" Molecules 18, no. 6: 6504-6520. https://doi.org/10.3390/molecules18066504