Abstract
The apical sodium-dependent bile salt transporter (ASBT) plays a pivotal role in maintaining bile acid homeostasis. Inhibition of ASBT would reduce bile acid pool size and lower cholesterol levels. In this report, a series of novel arylsulfonylaminobenzanilides were designed and synthesized as potential inhibitors of ASBT. Most of them demonstrated great potency against ASBT’s bile acid transport activity. In particular, compound 5g2 inhibited ASBT activity with an IC50 value of 0.11 μM. These compounds represent potential cholesterol-lowering drugs.
1. Introduction
Coronary artery disease (CAD) is a leading cause of death around the World [1]. High levels of cholesterol are one of the main causes of CAD, which greatly increases the risk of formation of plaques and atherosclerosis [2]. As a result, lowering cholesterol is beneficial to the prevention of CAD. Bile acids are metabolites of cholesterol. Bile acids are synthesized in the liver and released into the duodenum after a meal to facilitate lipid absorption. In the ileum, most bile acids are reabsorbed by the apical sodium-dependent bile salt transporter (ASBT, SLC10A2) and transported back to the liver through enterohepatic circulation. However, there is a fraction of bile acids that escape intestinal reabsorption, and are excreted with feces. The loss of bile acid in feces triggers de novo bile acids synthesis from cholesterol in order to maintain the bile acid pool size. This process represents the major route for the elimination of cholesterol from the body [3].
The argument that the increase of bile acids excretion can reduce hepatic and serum cholesterol was proven by the usage of bile acid sequestrants (BASs) [4]. As one of the most commonly used drugs for treating hypercholesterolemia and hyperlipidemia, BASs bind to bile acids and prevent their re-absorption in the intestine. Although BASs have a good safety record and synergistic effects when combined with statins, they still suffer from poor patient compliance due to their high dosages and bad palatability [5]. Therefore, the development of new drugs with similar physiological response to BASs, but with improved palatability, is in demand for lowering cholesterol.
ASBT plays a critical role in maintaining the bile acids pool size by reabsorbing bile acids in the ileum [6,7,8]. Ablation of ASBT function reduces bile acid pool size in mouse. Lower serum cholesterol levels were also observed in humans with ASBT mutations [9]. Therefore, ASBT is an attractive target for developing new cholesterol-lowering drugs [10]. Inhibition of ASBT function can increase bile acid fecal loss, which in turn stimulates hepatic conversion of cholesterol into bile acids [11]. Because ASBT is localized on the apical membrane of the lumen in the ileum, its inhibitors can block ASBT activity without entering the circulation system. This non-systemic character of ASBT inhibitors implies a low risk of potential systemic toxicity and drug–drug interactions [12,13]. So far, a number of ASBT inhibitors having various structural characteristics have been synthesized. Among of them, three candidates—264W94, SC-435 and R-146224 (Figure 1) were reported to block bile acid re-absorption and reduce cholesterol levels significantly in animal models [14,15,16]. In addition, it has recently been demonstrated in a Phase Ⅲ trial that A3309 (Figure 1), another ASBT inhibitor, can be used to treat patients with chronic idiopathic constipation (CIC).
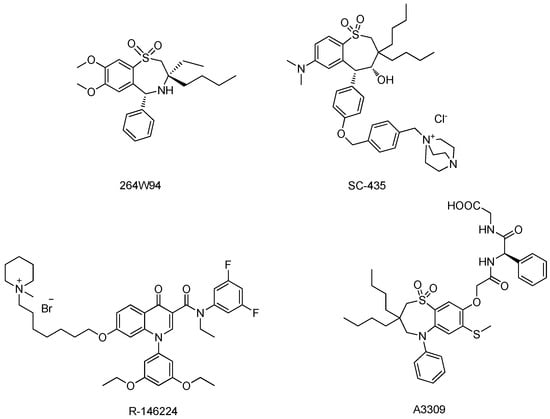
Figure 1.
Structures of ASBT inhibitors.
Baringhaus et al. developed a reliable 3D QSAR pharmacophore model for ASBT and screened a novel compound S-1647 (Figure 2) with considerable inhibition against ASBT (IC50: 4 μM) [17]. The simpler structure of S-1647 containing the three benzene rings A, B and C, compared with 264W94, SC-435 and R-146224, attracted our attention. We decided to make structural modifications on S-1647. In this study structure–activity relationships (SAR) of the relative positions of the ring C carbamyl group to ring B were investigated first, leading to three classes of compounds, and then various substitutions of rings A and C were added (Figure 2). Our primary objective was to optimize the potency of S-1647 against ASBT and a preliminary SAR was also explored to facilitate the further study of this class of compounds.
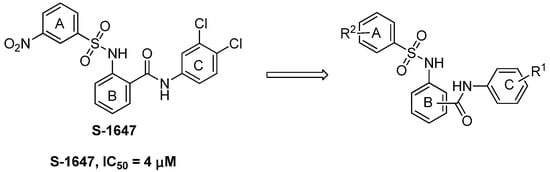
Figure 2.
Design of arylsulfonylaminobenzanilides.
2. Result and Discussion
2.1. Chemistry
The synthetic pathways to this series of target compounds were shown in Scheme 1. Nucleophilic substitution of substituted sulfonyl chlorides 1a–e with various aminobenzoates 2a–c in the presence of pyridine in tetrahydrofuran (THF) gave arylsulfonylaminobenzoates 3a–g. Hydrolysis of the benzoates 3a–g in a NaOH-H2O-EtOH system yielded the corresponding arylsulfonylaminobenzoic acids 4a–g. Coupling of the benzoic acids 4a–g with commercially available substituted anilines in the presence of 1-hydroxybenzotrizole (HOBt), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydro- chloride (EDC.HCl) and ethyldiisopropylamine (DIEA) in dimethylformamide (DMF) afforded the target compounds 5a–g.
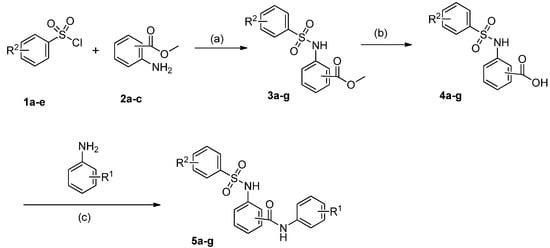
Scheme 1.
The synthesis of arylsulfonylamino-benzanilides 5a–g.
2.2. Biological Activity
In vitro inhibitory activity of all target compounds against ASBT was evaluated using a radioisotope-based assay. All the newly synthesized derivatives were initially tested at 10 μM concentration (Table 1).

Table 1.
The structures and ASBT inhibitory rate of 5a1–a4, 5b1–b3 and 5c1–c2. 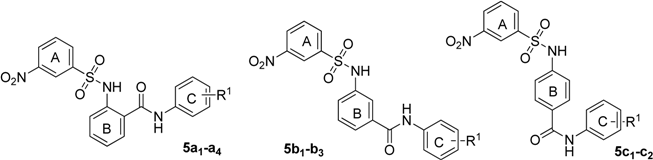

| Compd. | R1 | Inhibition (%) a | Compd. | R1 | Inhibition (%) a |
|---|---|---|---|---|---|
| 5a1 (S-1647) | 3,4-dichloro | 79.3 ± 5.2 | 5b2 | 3-difluoromethoxy | 30.6 ± 4.3 |
| 5a2 | 3-chloro-4-fluoro | 95.4 ± 3.3 | 5b3 | 3,4-dichloro | 62.4 ± 2.9 |
| 5a3 | 3-difluoromethoxy | 89.0 ± 2.6 | 5c1 | 3- trifluoromethoxy | 29.6 ± 3.5 |
| 5a4 | 3-trifluoromethoxy | 83.8 ± 3.4 | 5c2 | 3,4-dichloro | 34.8 ± 3.7 |
| 5b1 | 3-chloro-4-fluoro | 66.3 ± 5.0 |
a Values represent the percent inhibition of ASBT at 10 µM of the test compounds and are the average of three independent experiments.
The results suggest that the activity against ASBT decreased while the relative distance of the ring C carbamyl group to ring B increased. For example, ortho position compounds 5a1–a4 exhibited better activity than the corresponding meta position compounds 5b1–b3 and para position compounds 5c1–c2, so the carbamyl group in the ortho position with respect to the ring B is preferably for activity. Then, we explored the nitro group position in the ring A, and prepared two types of compounds (Table 2).

Table 2.
The structures and ASBT inhibitory rate of 5a5–a10 and 5d1–d6. 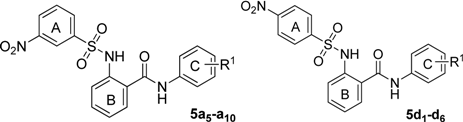

| Compd. | R1 | Inhibition (%) a | Compd. | R1 | Inhibition (%) a |
|---|---|---|---|---|---|
| 5a5 | 3,5-difluoro | 21.6 ± 4.1 | 5d1 | 2,4- dichloro | 66.9 ± 3.5 |
| 5a6 | 3-hydroxy-4-methoxy | 32.1 ± 3.3 | 5d2 | 3-chloro-4-fluoro | 75.5 ± 4.4 |
| 5a7 | 3,5-dichloro | 56.3 ± 2.8 | 5d3 | 3-trifluoromethyl-4-methyl | 73.7 ± 3.8 |
| 5a8 | 2,4-dichloro | 99.1 ± 2.3 | 5d4 | 3-difluoromethoxy | 58.8 ± 4.3 |
| 5a9 | 3- chloro | 46.9 ± 3.6 | 5d5 | 3- trifluoromethoxy | 66.7 ± 3.9 |
| 5a10 | 3-trifluoromethyl-4-methyl | 90.7 ± 4.2 | 5d6 | 3,4- dichloro | 73.9 ± 2.2 |
a Values represent the percent inhibition of ASBT at 10 µM of the test compounds and are the average of three independent experiments.
The position of the nitro group was considered an important factor in the activity. Apparently, compounds 5a1–a4, 5a8, and 5a10 with 3-nitro groups showed better activity than the corresponding 4-nitro analogues 5d1–d6, respectively. Finally, we investigated the R2 group in ring A, and designed three types of compounds (Table 3).

Table 3.
The structures and ASBT inhibitory rate of 5e1–e5, 5f1–f5 and 5g1–g5. 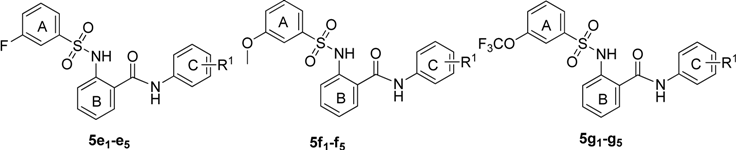

| Compd. | R1 | Inhibition (%) a | Compd. | R1 | Inhibition (%) a |
|---|---|---|---|---|---|
| 5e1 | 2,4-dichloro | 42.6 ± 3.4 | 5f4 | 3-trifluoromethoxy | 25.8 ± 2.1 |
| 5e2 | 3-chloro-4-fluoro | 63.2 ± 3.8 | 5f5 | 3,4-dichloro | 20.1 ± 1.9 |
| 5e3 | 3-trifluoromethyl-4-methyl | 57.6 ± 2.9 | 5g1 | 2,4-dichloro | 89.3 ± 3.3 |
| 5e4 | 3-trifluoromethoxy | 45.7 ± 3.6 | 5g2 | 3-chloro-4-fluoro | 98.6 ± 2.6 |
| 5e5 | 3,4-dichloro | 40.6 ± 2.8 | 5g3 | 3-trifluoromethyl-4-methyl | 91.8 ± 3.5 |
| 5f1 | 2,4-dichloro | 21.2 ± 3.0 | 5g4 | 3-trifluoromethoxy | 88.1 ± 2.7 |
| 5f2 | 3-chloro-4-fluoro | 59.5 ± 3.6 | 5g5 | 3,4-dichloro | 80.8 ± 4.3 |
| 5f3 | 3-trifluoromethyl-4-methyl | 43.0 ± 3.2 |
a Values represent the percent inhibition of ASBT at 10 µM of the test compounds and are the average of three independent experiments.
The electronic properties of the R2 group were also an important factor in the activity. Electron- withdrawing groups exhibited better activity than electron-donating groups. Compounds 5a, 5e and 5g showed better activity than 5f. Compounds where the R2 group was 3-trifluoromethoxy showed the best inhibitory effect.

Table 4.
IC50 values of target compounds. 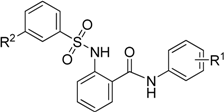

| Compd. | R1 | R2 | Inhibition (%) a | IC50 (µM) |
|---|---|---|---|---|
| 5a2 | 3-chloro-4-fluoro | 3-nitro | 95.4 ± 3.3 | 1.32 ± 0.28 |
| 5a3 | 3-difluoromethoxy | 3-nitro | 89.0 ± 2.6 | 2.84 ± 0.52 |
| 5a4 | 3-trifluoromethoxy | 3-nitro | 83.8 ± 3.4 | 1.23 ± 0.12 |
| 5a8 | 2,4-dichloro | 3-nitro | 99.1 ± 2.3 | 0.37 ± 0.08 |
| 5a10 | 3-trifluoromethyl-4-methyl | 3-nitro | 90.7 ± 4.2 | 1.49 ± 0.09 |
| 5g1 | 2,4-dichloro | 3-trifluoromethoxy | 89.3 ± 3.3 | 0.91 ± 0.18 |
| 5g2 | 3-chloro-4-fluoro | 3-trifluoromethoxy | 98.6 ± 2.6 | 0.11 ± 0.05 |
| 5g3 | 3-trifluoromethyl-4-methyl | 3-trifluoromethoxy | 91.8 ± 3.5 | 0.83 ± 0.11 |
| 5g4 | 3-trifluoromethoxy | 3-trifluoromethoxy | 88.1 ± 2.7 | 1.03 ± 0.16 |
| 5g5 | 3,4-dichloro | 3-trifluoromethoxy | 80.8 ± 4.3 | 1.22 ± 0.20 |
| S-1647 | 3,4-dichloro | 3-nitro | 79.3 ± 5.2 | 1.52 ± 0.12 |
a Values represent the percent inhibition of ASBT at 10 µM of the test compounds and are the average of three independent experiments.
Finally, the compounds with inhibition rate above 80% were further assessed based on IC50 values and the results are listed in Table 4. All of the tested compounds showed considerable activity (IC50: 0.11–2.84 μM). Among of them, 5a8, 5g1, 5g2 and 5g3 were more active than the lead compound S-1647. Particularly, compound 5g2 which had the best inhibitory activity (IC50: 0.11 μM) was found to be 14- fold more active than S-1647 (IC50: 1.52 μM).
3. Experimental
3.1. General
All melting points were obtained on a Büchi Melting Point B-540 apparatus (Büchi Labortechnik, Flawil, Switzerland) and are uncorrected. Mass spectra (MS) were taken in ESI mode on an Agilent 1100 LC-MS system (Agilent, Palo Alto, CA, USA). Nuclear magnetic resonance spectroscopy was performed using a 400 MHz Bruker ARX-400 spectrometers (Bruker Bioscience, Billerica, MA, USA) with DMSO-d6 as solvent and TMS as an internal standard. All the starting materials were obtained from commercially available sources and used without further purification, unless otherwise specified. Yields were not optimized.
Methyl 2-(3-Nitrophenylsulfonamido)benzoate (3a). To a solution of 1a (5.0 g, 21.4 mmol) in THF (60 mL) was added methyl 2-aminobenzoate (2a, 2.7 mL, 21.4 mmol) and then pyridine (1.7 mL, 21.4 mmol). The reaction mixture was stirred for 9 h at room temperature and then concentrated. To the residue was added water and then 5% HCl. The mixture was stirred for 0.5h and filtered. The filter cake was washed with water, dried and gave 3a as a red solid (74.0% yield); m.p.: 127.1–128.0 °C. 1H-NMR δ: 3.75 (3H, s), 7.25 (1H, t, J = 6.0 Hz), 7.39 (1H, d, J = 6.0 Hz), 7.57 (1H, t, J = 6.0 Hz), 7.79 (1H, dd, J1 = 6.0 Hz, J2 = 1.2 Hz), 7.85 (1H, t, J = 6.4 Hz), 8.15 (1H, d, J = 6.4 Hz), 8.44–8.47 (2H, m), 10.49 (1H, s). MS m/z: 359.07 [M+Na]+.
Methyl 3-(3-Nitrophenylsulfonamido)benzoate (3b). Compound 3b was obtained as a white solid (78.7% yield) from compounds 1a and 2b as described for 3a; m.p.: 153.5–154.4 °C. 1H-NMR δ: 3.82 (3H, s) , 7.37–7.45 (2H, m), 7.66 (1H, d, J = 7.6 Hz), 7.72 (1H, s), 7.86 (1H, t, J = 8.0 Hz), 8.13 (1H, d, J = 7.6 Hz), 8.45 (1H, dd, J1 = 8.0 Hz, J2= 2.0 Hz), 8.49 (1H, t, J = 2.0 Hz), 10.80 (1H, s). MS m/z: 335.16 [M-H]−.
Methyl 4-(3-Nitrophenylsulfonamido)benzoate (3c). Compound 3c was obtained as a white solid (76.3% yield) from compounds 1a and 2c as described for 3a; m.p.: 209.9–211.0 °C. 1H-NMR δ: 3.78 (3H, s), 7.25 (2H, d, J = 8.8 Hz), 7.84–7.89 (3H, m), 8.20 (1H, d, J = 8.0 Hz), 8.45 (1H, dd, J1 = 8.0 Hz, J2= 2.0 Hz), 8.54 (1H, t, J = 2.0 Hz), 11.10 (1H, s). MS m/z: 335.16 [M−H]−.
Methyl 2-(4-Nitrophenylsulfonamido)benzoate (3d). Compound 3d was obtained as a yellow solid (67.7% yield) from compounds 1b and 2a as described for 3a; m.p.: 154.9–155.7 °C. 1H-NMR δ: 3.76 (3H, s), 7.25 (1H, t, J = 6.0 Hz), 7.40 (1H, d, J = 6.8 Hz), 7.58 (1H, t, J = 6.0 Hz), 7.80 (1H, dd, J1 = 6.4 Hz, J2 = 0.8 Hz), 8.01 (2H, d, J = 7.2 Hz), 8.35 (2H, d, J = 7.2 Hz), 10.55 (1H, s). MS m/z: 335.08 [M−H]−.
Methyl 2-(3-Fluorophenylsulfonamido)benzoate (3e). Compound 3e was obtained as a white solid (72.6% yield) from compounds 1c and 2a as described for 3a; m.p.: 122.3–123.5 °C. 1H-NMR δ: 3.80 (3H, s), 7.22 (1H, t, J = 8.0 Hz), 7.43 (1H, d, J = 8.0 Hz), 7.51-7.63 (5H, m), 7.83 (1H, dd, J1 = 8.0 Hz, J2 = 1.6 Hz), 10.42 (1H, s). MS m/z: 310.95 [M+H]+.
Methyl 2-(3-Methoxyphenylsulfonamido)benzoate (3f). Compound 3f was obtained as a white solid (73.3% yield) from compounds 1d and 2a as described for 3a; m.p.: 123.7–124.9 °C. 1H-NMR δ: 3.76 (3H, s), 3.81 (3H, s), 7.17–7.24 (3H, m), 7.34 (1H, d, J = 7.6 Hz), 7.47 (2H, t, J = 8.0 Hz), 7.58 (1H, t, J = 8.0 Hz), 7.84 (1H, dd, J1 = 8.0 Hz, J2 = 1.6 Hz), 10.37 (1H, s). MS m/z: 320.05 [M−H]−.
Methyl 2-(3-(Trifluoromethoxy)phenylsulfonamido)benzoate (3g). To a solution of 1e (8.6 g, 33.1 mmol) in THF (60 mL) was added methyl 2-aminobenzoate (2a, 5.0 g, 33.1 mmol) and then pyridine (3.2 g, 39.7 mmol). The reaction mixture was stirred for 9 h at room temperature. To the residue was added water and then 5% HCl. The mixture was stirred for 0.5 h and extracted with dichloromethane. The dichloromethane layer was dried over MgSO4, concentrated in vacuo to afford 3g as a red liquid (75.2% yield) that was used directly for the next reaction without further purification.
2-(3-Nitrophenylsulfonamido)benzoic acid (4a). To a solution of 3a (5.7 g, 17.0 mmol) in the ethanol (20 mL) was added 10% aqueous sodium hydroxide (12 mL). The mixture was heated to 80 °C for 8 h and cooled to room temperature. The solution was concentrated and dissolved in water (50 mL). The mixture was adjusted to pH 2 with 6 N hydrochloric acid to give a white precipitate. The precipitate was filtered and washed with water to pH 7. The filter cake was dried to give 4a (86.2% yield) as a white solid; m.p.: 219.8–220.5 °C. 1H-NMR δ: 7.17 (1H, t, J = 7.6 Hz), 7.47 (1H, d, J = 8.0 Hz), 7.56 (1H, t, J = 8.4 Hz), 7.82–7.88 (2H, m), 8.19 (1H, d, J = 7.6 Hz), 8.45–8.47 (2H, m), 11.28 (1H, s). MS m/z: 321.08 [M−H]−.
3-(3-Nitrophenylsulfonamido)benzoic acid (4b). Obtained as a white solid (86.1% yield) from compound 3b as described for 4a; m.p.: 249.0–250.2 °C. 1H-NMR δ: 7.34–7.41 (2H, m), 7.63 (1H, d, J = 7.2 Hz), 7.69 (1H, s), 7.86 (1H, t, J = 8.0 Hz), 8.12 (1H, d, J = 8.0 Hz), 8.45 (1H, dd, J1 = 8.0 Hz, J2 = 2.0 Hz,), 8.49 (1H, t, J = 2.0 Hz), 10.80 (1H, s), 13.10 (1H, s). MS m/z: 321.07 [M−H]−.
4-(3-Nitrophenylsulfonamido)benzoic acid (4c). Obtained as a white solid (83.9% yield) from compound 3c as described for 4a; m.p.: 269.4–270.6 °C. 1H-NMR δ: 7.22 (2H, d, J = 8.8 Hz), 7.81–7.89 (3H, m), 8.20 (1H, d, J = 8.0 Hz), 8.45 (1H, dd, J1 = 8.0 Hz, J2= 2.0 Hz), 8.54 (1H, t, J = 2.0 Hz), 11.06 (1H, s), 12.80 (1H, s). MS m/z: 321.08 [M−H]−.
2-(4-Nitrophenylsulfonamido)benzoic acid (4d). Obtained as a yellow solid (82.5% yield) from compound 3d as described for 4a; m.p.: 238.1–239.2 °C. 1H-NMR δ: 7.16 (1H, t, J = 6.0 Hz), 7.47 (1H, d, J = 6.4 Hz), 7.55 (1H, t, J = 6.8 Hz), 7.89 (1H, dd, J1 = 6.0 Hz, J2 = 1.2 Hz), 8.05 (2H, d, J = 7.2 Hz), 8.34 (2H, d, J = 7.2 Hz), 10.39 (1H, s). MS m/z: 321.00 [M−H]−.
2-(3-Fluorophenylsulfonamido)benzoic acid (4e). Obtained as a white solid (88.3% yield) from compound 3e as described for 4a; m.p.: 187.1–188.2 °C. 1H-NMR δ: 7.16 (1H, t, J = 8.0 Hz), 7.48 (1H, d, J = 8.0 Hz), 7.51–7.66 (5H, m), 7.90 (1H, dd, J1 = 8.0 Hz, J2 = 1.6 Hz), 11.22 (1H, s). MS m/z: 294.09 [M−H]−.
2-(3-Methoxyphenylsulfonamido)benzoic acid (4f). Obtained as a white solid (82.7% yield) from compound 3f as described for 4a; m.p.: 157.1–158.3 °C. 1H-NMR δ: 3.76 (3H, s), 7.12 (1H, t, J = 8.0 Hz), 7.20 (1H, dd, J1 = 8.0 Hz, J2 = 2.4 Hz), 7.26 (1H, t, J = 2.0 Hz), 7.35 (1H, d, J = 8.0 Hz), 7.47 (1H, t, J = 8.0 Hz), 7.52-7.58 (2H, m), 7.89 (1H, dd, J1 = 8.0 Hz, J2 = 1.6 Hz), 11.08 (1H, s), 13.97 (1H, s). MS m/z: 306.12 [M−H]−.
2-(3-(Trifluoromethoxy)phenylsulfonamido)benzoic acid (4g). Obtained as a white solid (85.1% yield) from compound 3g as described for 4a; m.p.: 135.0–136.2 °C. 1H-NMR δ: 7.17 (1H, t, J = 8.0 Hz), 7.50 (1H, d, J = 8.0 Hz), 7.57 (1H, t, J = 8.0 Hz), 7.67-7.74 (3H, m), 7.84 (1H, d, J = 7.6 Hz), 7.89 (1H, dd, J1 = 7.6 Hz, J2 = 1.2 Hz), 11.17 (1H, s). MS m/z: 360.11 [M−H]−.
3.2. General Procedure for the Synthesis of Arylsulfonylaminobenzanilides
To a solution of 4a–g (1 mmol) in dry DMF (10 mL) was added HOBt (1.5 mmol) and EDC.HCl (1.5 mmol). The reaction mixture was stirred at room temperature for 2 h, and then the substituted arylamines (2.0 mmol) and DIEA (2.0 mmol) were added, and then stirred at room temperature for 12 h, poured into ice-cold water. The precipitate was filtered, washed with water, and then recrystallized with ethyl acetate or purified by column chromatography (silica gel) to give the title compounds.
N-(3,4-Dichlorophenyl)-2-(3-nitrophenylsulfonamido)benzamide (5a1). White solid, 76.7% yield, m.p.: 185.0–186.3 °C. 1H-NMR δ: 7.30–7.34 (2H, m), 7.48–7.51 (2H, m), 7.57–7.62 (2H, m), 7.73 (1H, t, J = 8.0 Hz), 7.95 (1H, s), 8.07 (1H, d, J = 7.6 Hz), 8.32 (1H, d, J = 8.0 Hz), 8.39 (1H, s), 10.34 (1H, s), 10.41 (1H, s). MS m/z: 464.18 [M−H]−.
N-(3-Chloro-4-fluorophenyl)-2-(3-nitrophenylsulfonamido)benzamide (5a2). White solid, 80.6% yield, m.p.: 203.4–204.1 °C. 1H-NMR δ: 7.33–7.40 (3H, m), 7.45–7.53 (2H, m), 7.61 (1H, d, J = 7.2 Hz), 7.72 (1H, t, J =8.0 Hz), 7.87 (1H, dd, J1 = 6.8 Hz, J2 = 2.4 Hz), 8.06 (1H, d, J = 8.4 Hz), 8.32 (1H, dd, J1 = 8.4 Hz, J2 = 1.6 Hz), 8.38 (1H, t, J =4.0 Hz), 10.35 (1H, s), 10.38 (1H, s). MS m/z: 448.07 [M−H]−.
N-(3-(Difluoromethoxy)phenyl)-2-(3-nitrophenylsulfonamido)benzamide (5a3). Yellow solid, 43.3% yield, m.p.: 127.4–128.2 °C. 1H-NMR δ: 6.91 (1H, d, J = 8.0 Hz), 7.19 (1H, s), 7.31–7.42 (4H, m), 7.49–7.53 (2H, m), 7.63 (1H, d, J = 7.6 Hz), 7.71 (1H, t, J = 8.0 Hz), 8.08 (1H, d, J = 8.0 Hz), 8.31 (1H, dd, J1 = 8.0 Hz, J2 = 1.2 Hz), 8.38 (1H, t, J = 2.0 Hz), 10.33 (1H, s), 10.41 (1H, s). MS m/z: 462.15 [M−H]−.
2-(3-Nitrophenylsulfonamido)-N-(3-(trifluoromethoxy)phenyl)benzamide (5a4). White solid, 47.6% yield, m.p.: 141.4–142.0 °C. 1H-NMR δ: 7.08 (1H, d, J = 8.0 Hz), 7.33 (2H, t, J = 8.0 Hz), 7.44 (1H, t, J = 8.0 Hz), 7.49–7.54 (2H, m), 7.63 (1H, d, J = 7.6 Hz), 7.70 (2H, t, J = 8.0 Hz), 8.07 (1H, d, J = 7.6 Hz), 8.31 (1H, dd, J1 = 8.4 Hz, J2 = 1.6 Hz), 8.39 (1H, t, J = 2.0 Hz), 10.37 (1H, s), 10.42 (1H, s). MS m/z: 504.12 [M+Na]+.
N-(3,5-Difluorophenyl)-2-(3-nitrophenylsulfonamido)benzamide (5a5). White solid, 78.0% yield, m.p.: 181.1–181.9 °C. 1H-NMR δ: 7.01 (1H, d, J = 6.0 Hz), 7.23 (1H, t, J = 6.0 Hz), 7.35–7.41 (3H, m), 7.44–7.46 (1H, m), 7.48–7.52 (2H, m), 8.03 (1H, t, J = 6.4 Hz), 8.45 (1H, d, J = 6.4 Hz), 8.67 (1H, dd, J1 = 6.8 Hz, J2 = 1.2 Hz), 8.73 (1H, s), 10.24 (1H, s). MS m/z: 432.12 [M−H]−.
N-(3-Hydroxy-4-methoxyphenyl)-2-(3-nitrophenylsulfonamido)benzamide (5a6). Yellow solid, 65.1% yield, m.p.: 168.9–170.1 °C. 1H-NMR δ: 3.30 (3H, s), 7.01 (1H, d, J = 6.0 Hz), 7.23 (1H, t, J = 6.0 Hz), 7.35–7.40 (3H, m), 7.44–7.47 (1H, m), 7.49–7.52 (2H, m), 8.03 (1H, t, J = 6.4 Hz), 8.45 (1H, d, J = 6.4 Hz), 8.67 (1H, d J = 6.4 Hz), 8.73 (1H, s), 10.24 (1H, s). MS m/z: 442.08 [M−H]−.
N-(3,5-Dichlorophenyl)-2-(3-nitrophenylsulfonamido)benzamide (5a7). White solid, 72.3% yield, m.p.: 209.9–210.4 °C. 1H-NMR δ: 7.30–7.36 (3H, m), 7.51 (1H, t, J = 8.0 Hz), 7.60 (1H, d, J = 7.2 Hz), 7.64 (2H, d, J = 2.0 Hz), 7.73 (1H, t, J = 8.0 Hz), 8.06 (1H, d, J = 8.4 Hz), 8.31-8.33 (1H, m), 8.40-8.41 (1H, m), 10.31 (1H, s), 10.45 (1H, s). MS m/z: 464.07 [M−H]−.
N-(2,4-Dichlorophenyl)-2-(3-nitrophenylsulfonamido)benzamide (5a8). White solid, 49.6% yield, m.p.: 203.6–204.1 °C. 1H-NMR δ: 7.30–7.35 (2H, m), 7.49–7.59 (3H,m), 7.72 (1H, d, J = 2.0 Hz),7.83 (1H, t, J = 8.0 Hz), 7.93 (1H, d, J = 8.0 Hz), 8.14 (1H, dd, J1 = 8.0 Hz, J2 = 2.0 Hz), 8.38 (1H, t, J = 2.0 Hz), 8.45 (1H, d, J = 8.0 Hz), 10.24 (1H, s), 10.88 (1H, s). MS m/z: 464.10 [M−H]−.
N-(3-Chlorophenyl)-2-(3-nitrophenylsulfonamido)benzamide (5a9). White solid, 70.2% yield, m.p.: 167.3–168.1 °C. 1H-NMR δ: 7.15 (1H, d, J = 8.0 Hz), 7.31–7.35 (3H, m), 7.46 (1H, d, J = 8.4 Hz), 7.50 (1H, t, J = 8.0 Hz), 7.63 (1H, d, J = 7.6 Hz), 7.72 (1H, t, J = 8.0 Hz), 7.77 (1H, s), 8.07 (1H, d, J = 8.0 Hz), 8.32 (1H, dd, J1 = 8.0 Hz, J2 = 1.6 Hz), 8.39 (1H, t, J = 2.4 Hz), 10.33 (1H, s), 10.40 (1H, s). MS m/z: 430.09 [M−H]−.
N-(4-Methyl-3-(trifluoromethyl)phenyl)-2-(3-nitrophenylsulfonamido)benzamide (5a10). White solid, 40.3% yield, m.p.: 166.4–167.4 °C 1H-NMR δ: 2.40 (3H, s), 7.31–7.38 (3H, m), 7.51 (1H, t, J = 7.6 Hz), 7.64 (1H, d, J = 7.6 Hz), 7.70 (2H, t, J = 8.0 Hz), 7.97 (1H, d, J = 1.2 Hz), 8.06 (1H, d, J = 8.0 Hz), 8.27–8.30 (1H, m), 8.37 (1H, t, J = 2.0 Hz), 10.36 (1H, s), 10.42 (1H, s). MS m/z: 478.10 [M−H]−.
N-(3-Chloro-4-fluorophenyl)-3-(3-nitrophenylsulfonamido)benzamide (5b1). White solid, 55.3% yield, m.p.: 161.3–162.6 °C. 1H-NMR δ: 7.31–7.33 (1H, m), 7.37–7.45 (2H, m), 7.63–7.67 (3H, m), 7.86 (1H, t, J = 8.0 Hz), 8.01 (1H, dd, J1 = 7.2 Hz, J2= 2.8 Hz), 8.13–8.15 (1H, m), 8.43–8.46 (1H, m), 8.51 (1H, t, J = 2.0 Hz), 10.41 (1H, s), 10.80 (1H, s). MS m/z: 448.75 [M−H]−.
N-(3-(Difluoromethoxy)phenyl)-3-(3-nitrophenylsulfonamido)benzamide (5b2). White solid, 57.7% yield, m.p.: 159.8–160.6 °C. 1H-NMR δ: 6.90 (1H, d, J = 8.0 Hz), 7.19 (1H, s), 7.31–7.44 (3H, m), 7.59 (1H, d, J = 8.0 Hz), 7.66 (3H, d, J = 8.0 Hz), 7.85 (1H, t, J = 8.0 Hz), 8.15 (1H, d, J = 8.0 Hz), 8.45 (1H, d, J = 8.0 Hz), 8.51 (1H, s), 10.39 (1H, s), 10.80 (1H, s). MS m/z: 462.49 [M−H]−.
N-(3,4-dichlorophenyl)-3-(3-nitrophenylsulfonamido)benzamide (5b3). Yellow solid, 60.8% yield, m.p.: 192.5–193.7 °C. 1H-NMR δ: 7.32 (1H, d, J = 8.0 Hz), 7.43 (1H, t, J = 8.0 Hz), 7.59–7.70 (4H, m), 7.85 (1H, t, J = 8.0 Hz), 8.09 (1H, d, J = 2.4 Hz), 8.15 (1H, d, J = 8.0 Hz), 8.45 (1H, d, J = 8.0 Hz), 8.51 (1H, s), 10.48 (1H, s), 10.80 (1H, s). MS m/z: 464.91 [M−H]−.
4-(3-Nitrophenylsulfonamido)-N-(3-(trifluoromethoxy)phenyl)benzamide (5c1). White solid, 51.3% yield, m.p.: 177.3–178.2 °C. 1H-NMR δ: 7.09 (1H, d, J = 8.8 Hz), 7.26 (2H, d, J = 8.0 Hz), 7.46 (1H, t, J = 8.0 Hz), 7,71 (1H, d, J = 8.0 Hz), 7.85–7.91 (4H, m), 8.23 (1H, d, J = 8.0 Hz), 8.47 (1H, dd, J1 = 8.0 Hz, J2= 2.0 Hz), 8.57 (1H, s), 10.37 (1H, s), 11.04 (1H, s). MS m/z: 480.57 [M−H]−.
N-(3,4-Dichlorophenyl)-4-(3-nitrophenylsulfonamido)benzamide (5c2). White solid, 57.8% yield, m.p.: 189.2–190.5 °C. 1H-NMR δ: 7.25 (2H, d, J = 8.0 Hz), 7.59 (1H, d, J = 8.8 Hz), 7.69 (1H, dd, J1 = 8.8 Hz, J2= 2.4 Hz), 7.83–7.90 (3H, m), 8.09 (1H, d, J = 2.0 Hz), 8.22 (1H, d, J = 8.0 Hz), 8.47 (1H, dd, J1 = 8.0 Hz, J2= 2.0 Hz), 8.56 (1H, s), 10.34 (1H, s), 11.04 (1H, s). MS m/z: 464.65 [M−H]−.
N-(2,4-Dichlorophenyl)-2-(4-nitrophenylsulfonamido)benzamide (5d1). White solid, 76.1% yield, m.p.: 220.6–221.3 °C. 1H-NMR δ: 7.31–7.37 (2H, m), 7.46–7.57 (3H, m), 7.72 (1H, d, J = 2.0 Hz), 7.97 (2H, d, J = 8.8 Hz), 8.33 (3H, m), 10.25 (1H, s), 10.96 (1H, s). MS m/z: 464.08 [M−H]−.
N-(3-Chloro-4-fluorophenyl)-2-(4-nitrophenylsulfonamido)benzamide (5d2). White solid, 77.5% yield, m.p.: 226.4–227.1 °C. 1H-NMR δ: 7.35–7.40 (3H, m), 7.48–7.54 (2H, m), 7.64 (1H, d, J = 7.2 Hz), 7.85 (1H, dd, J1 = 7.2 Hz, J2 = 2.4 Hz), 7.91 (2H, d, J = 8.8 Hz), 8.21 (2H, d, J = 8.8 Hz), 10.33 (1H, s), 10.39 (1H, s). MS m/z: 448.07 [M−H]−.
N-(4-Methyl-3-(trifluoromethyl)phenyl)-2-(4-nitrophenylsulfonamido)benzamide (5d3). White solid, 53.7% yield, m.p.: 198.8–199.6 °C. 1H-NMR δ: 2.41 (3H, s), 7.33–7.40 (3H, m), 7.52–7.56 (1H, m), 7.67 (1H, d, J = 7.6 Hz), 7.74 (1H, d, J = 7.6 Hz),7.90 (2H, d, J = 8.8 Hz), 7.95 (1H, s), 8.18 (2H, d, J = 8.8 Hz), 10.36 (1H, s), 10.43 (1H, s). MS m/z: 478.21 [M−H]−.
N-(3-(Difluoromethoxy)phenyl)-2-(4-nitrophenylsulfonamido)benzamide (5d4). White solid, 69.1% yield, m.p.: 203.9–205 °C. 1H-NMR δ: 6.91 (1H, d, J = 8.8 Hz), 7.19 (1H, s), 7.33–7.41 (3H, m), 7.49–7.54 (2H, m), 7.65 (1H, d, J = 7.2 Hz), 7.92 (2H, d, J = 8.8 Hz), 8.21 (2H, d, J = 8.8 Hz), 10.33 (1H, s), 10.44 (1H, s). MS m/z: 462.15 [M−H]−.
2-(4-Nitrophenylsulfonamido)-N-(3-(trifluoromethoxy)phenyl)benzamide (5d5). White solid, 70.3% yield, m.p.: 198.8–199.4 °C. 1H-NMR δ: 7.08 (1H, d, J = 7.6 Hz), 7.33 (2H, d, J = 8.0 Hz), 7.44 (1H, t, J = 8.0 Hz), 7.53 (2H, d, J = 8.0 Hz), 7.65 (1H, d, J = 7.2 Hz), 7.73 (1H,s), 7.91 (2H, d, J = 8.8 Hz), 8.20 (2H, d, J = 8.8 Hz), 10.38 (1H, s), 10.40 (1H, s). MS m/z: 480.21 [M−H]−.
N-(3,4-Dichlorophenyl)-2-(4-nitrophenylsulfonamido)benzamide (5d6). White solid, 65.2% yield, m.p.: 243.4–243.6 °C. 1H-NMR δ: 7.32–7.34 (2H, m), 7.52 (1H, dd, J1 = 8.4 Hz, J2 = 2.4 Hz), 7.56 (2H, t, J = 8.8 Hz), 7.62 (1H, d, J = 7.2 Hz), 7.90 (2H, d, J = 8.8 Hz),7.92 (1H,s), 8.21 (2H, d, J = 8.8 Hz), 10.35 (1H, s), 10.39 (1H, s). MS m/z: 464.08 [M−H]−.
N-(2,4-Dichlorophenyl)-2-(3-fluorophenylsulfonamido)benzamide (5e1). White solid, 67.6% yield, m.p.: 146.3–147.6 °C. 1H-NMR δ: 7.29 (1H, t, J = 7.2 Hz), 7.40 (1H, d, J = 8.0 Hz), 7.50–7.64 (7H, m), 7.75 (1H, d, J = 2.0 Hz), 7.88 (1H, d, J = 8.0 Hz), 10.34 (1H, s), 10.90 (1H, s). MS m/z: 438.05 [M−H]−.
N-(3-Chloro-4-fluorophenyl)-2-(3-fluorophenylsulfonamido)benzamide (5e2). White solid, 52.1% yield, m.p.: 178.6–179.9 °C. 1H-NMR δ: 7.29 (1H, t, J = 7.6 Hz), 7.35 (1H, d, J = 8.0 Hz), 7.41–7.59 (7H, m), 7.70 (1H, d, J = 7.6 Hz), 7.95 (1H, dd, J1 = 6.8 Hz, J2= 2.4 Hz), 10.41 (1H, s), 10.47 (1H, s). MS m/z: 421.28 [M−H]−.
2-(3-Fluorophenylsulfonamido)-N-(4-methyl-3-(trifluoromethyl)phenyl)benzamide (5e3). Yellow solid, 55.8% yield, m.p.: 168.8–169.6 °C. 1H-NMR δ: 2.41 (3H, s), 7.27 (1H, t, J = 7.3 Hz), 7.35 (1H, d, J = 8.4 Hz), 7.42 (2H, d, J = 8.1 Hz), 7.48–7.56 (3H, m), 7.72 (1H, d, J = 6.8 Hz), 7.78 (1H, d, J = 8.0 Hz), 8.03 (1H, s), 10.46 (1H, s), 10.48 (1H, s). MS m/z: 451.30 [M−H]−.
2-(3-Fluorophenylsulfonamido)-N-(3-(trifluoromethoxy)phenyl)benzamide (5e4). White solid, 61.7% yield, m.p.: 166.8–168.7 °C. 1H-NMR δ: 7.11 (1H, d, J = 8.4 Hz), 7.26–7.34 (2H, m), 7.39–7.57 (6H, m), 7.62 (1H, d, J = 8.4 Hz), 7.70 (1H, d, J = 7.2 Hz), 7.79 (1H, s), 10.37 (1H, s), 10.52 (1H, s). MS m/z: 453.68 [M−H]−.
N-(3,4-Dichlorophenyl)-2-(3-fluorophenylsulfonamido)benzamide (5e5). White solid, 58.5% yield, m.p.: 179.1–180.6 °C. 1H-NMR δ: 7.27–7.33 (2H, m), 7.42–7.58 (5H, m), 7.60–7.64 (2H, m), 7.69 (1H, d, J = 8.0 Hz), 8.03 (1H, d, J = 1.6 Hz), 10.34 (1H, s), 10.53 (1H, s). MS-ESI m/z: 437.34 [M−H]−.
N-(2,4-Dichlorophenyl)-2-(3-methoxyphenylsulfonamido)benzamide (5f1). White solid, 51.5% yield, m.p.: 138.8–139.7 °C. 1H-NMR δ: 3.73 (3H, s), 7.19–7.30 (4H, m), 7.43–7.54 (4H, m), 7.61 (1H, d, J = 8.4 Hz), 7.75 (1H, d, J = 1.6 Hz), 7.88 (1H, d, J = 8.0 Hz), 10.35 (1H, s), 10.85 (1H, s). MS m/z: 449.44 [M−H]−.
N-(3-Chloro-4-fluorophenyl)-2-(3-methoxyphenylsulfonamido)benzamide (5f2). White solid, 57.8% yield, m.p.: 181.9–183.2 °C. 1H-NMR δ: 3.67 (3H, s), 7.12 (1H, dd, J1 = 8.0 Hz, J2= 2.4 Hz), 7.25–7.29 (2H, m), 7.36–7.45 (3H, m), 7.51 (1H, t, J = 7.6 Hz), 7.56–7.60 (1H, m), 7.71 (1H, d, J = 7.6 Hz), 7.94 (1H, dd, J1 = 6.8 Hz, J2= 2.4 Hz), 10.36 (1H, s), 10.46 (1H, s). MS m/z: 433.65 [M−H]−.
2-(3-Methoxyphenylsulfonamido)-N-(4-methyl-3-(trifluoromethyl)phenyl)benzamide (5f3).White solid, 56.2% yield, m.p.: 165.7–166.6 °C. 1H-NMR δ: 2.42 (3H, s), 3.63 (3H, s), 7.11 (1H, d, J = 8.0 Hz), 7.21 (1H, s), 7.26 (2H, t, J = 8.7 Hz), 7.35–7.44 (3H, m), 7.51 (1H, t, J = 7.7 Hz), 7.74 (1H, d, J = 7.7 Hz), 7.80 (1H, d, J = 8.0 Hz), 8.04 (1H, s), 10.42 (1H, s), 10.49 (1H, s). MS m/z: 463.58 [M−H]−.
2-(3-Methoxyphenylsulfonamido)-N-(3-(trifluoromethoxy)phenyl)benzamide (5f4). White solid, 63.5% yield, m.p.: 149.3–150.8 °C. 1H-NMR δ: 3.64 (3H, s), 7.09–7.27 (5H, m), 7.34–7.41 (2H, m), 7.46–7.53 (2H, m), 7.63 (1H, d, J = 7.6 Hz), 7.71 (1H, d, J = 7.6 Hz), 7.78 (1H, s), 10.33 (1H, s), 10.52 (1H, s). MS m/z: 465.22 [M−H]−.
N-(3,4-Dichlorophenyl)-2-(3-methoxyphenylsulfonamido)benzamide (5f5). White solid, 67.9% yield, m.p.: 160.3–161.4 °C. 1H-NMR δ: 3.66 (3H, s), 7.11 (1H, dd, J1 = 8.4 Hz, J2= 2.4 Hz), 7.28–7.30 (3H, m), 7.35–7.39 (2H, m), 7.50 (1H, d, J = 8.0 Hz), 7.57–7.63 (2H, m), 7.69 (1H, d, J = 8.0 Hz), 8.01 (1H, d, J = 2.0 Hz), 10.27 (1H, s), 10.51 (1H, s). MS m/z: 449.57 [M−H]−.
N-(2,4-Dichlorophenyl)-2-(3-(trifluoromethoxy)phenylsulfonamido)benzamide (5g1). White solid, 69.6% yield, m.p.: 151.6–152.8 °C. 1H-NMR δ: 7.30 (1H, t, J = 7.6 Hz), 7.35 (1H, d, J = 8.0 Hz), 7.49–7.51 (2H, m), 7.62–7.78 (6H, m), 7.88 (1H, d, J = 7.6 Hz), 10.31 (1H, s),10.94 (1H, s). MS m/z: 503.12 [M−H]−.
N-(3-Chloro-4-fluorophenyl)-2-(3-(trifluoromethoxy)phenylsulfonamido)benzamide (5g2). White solid, 68.1% yield, m.p.: 157.3–158.6 °C. 1H-NMR δ: 7.28–7.34 (2H, m), 7.42 (1H, t, J = 8.8 Hz), 7.50 (1H, t, J = 8.0 Hz), 7.54–7.74 (6H, m), 7.96 (1H, dd, J1 = 6.8 Hz, J2= 2.4 Hz), 10.46 (2H, s). MS m/z: 487.85 [M−H]−.
N-(4-Methyl-3-(trifluoromethyl)phenyl)-2-(3-(trifluoromethoxy)phenylsulfonamido)benzamide (5g3). White solid, 55.7% yield, m.p.: 179.4–180.5 °C. 1H-NMR δ: 2.42 (3H, s), 7.31 (1H, d, J = 7.6 Hz), 7.35 (1H, d, J = 8.4 Hz), 7.42 (1H, d, J = 8.4 Hz), 7.51 (1H, t, J = 8.0 Hz), 7.56–7.62 (2H, m), 7.64 (1H, s), 7.70–7.73 (2H, m), 7.77 (1H, d, J = 8.4 Hz), 8.06 (1H, s), 10.47 (1H, s), 10.51 (1H, s). MS m/z: 517.52 [M−H]−.
N-(3-(Trifluoromethoxy)phenyl)-2-(3-(trifluoromethoxy)phenylsulfonamido)benzamide (5g4). White solid, 62.3% yield, m.p.: 169.1–170.4 °C. 1H-NMR δ: 7.11 (1H, d, J = 8.4 Hz), 7.29–7.34 (2H, m), 7.46–7.51 (2H, m), 7.57–7.62 (3H, m), 7.66 (1H, s), 7.71 (2H, t, J = 7.2 Hz), 7.81 (1H, s), 10.43 (1H, s), 10.52 (1H, s). MS m/z: 519.12 [M−H]−.
N-(3,4-Dichlorophenyl)-2-(3-(trifluoromethoxy)phenylsulfonamido)benzamide (5g5). White solid, 66.7% yield, m.p.: 160.5–161.6 °C. 1H-NMR δ: 7.32 (2H, t, J = 8.0 Hz), 7.51 (1H, t, J = 8.0 Hz), 7.58–7.74 (7H, m), 8.05 (1H, d, J = 2.0 Hz), 10.40 (1H, s), 10.53 (1H, s). MS m/z: 503.75 [M−H]−.
3.3. ASBT Inhibition Assay
HEK293T was obtained from the American Type Culture Collection (Manassas, VA, USA), and grown in MEM supplemented with 100 U/mL penicillin and 100 mg/mL streptomycin and 10% heat-inactivated fetal bovine serum. Human ASBT expression construct was prepared as previously described [18]. HEK293T cells were seeded in 12 well plates and transiently transfected with 0.5 μg/well pcDNA3.1/ASBT or negative control plasmid pcDNA3.1 using lipofactamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Twenty-four hours after transfection, HEK293T cells were assayed for taurocholic acid uptake as previously described with minor modifications [19]. Briefly, cells were washed twice with warm wash & uptake buffer (116 mM NaCl, 5.3 mM KCl, 1.1 mM KH2PO4, 0.8 mM MgSO4, 1.8 mM CaCl2, 11 mM D-dextrose/D-glucose, and 10 mM HEPES, pH 7.4), then cells were incubated with the same buffer containing the indicated concentrations of test compounds (dissolved in dimethyl sulfoxide) and 1 μCi/mL of [3H]-taurocholic acid(TCA, 0.2 μM) (PerkinElmer Life Sciences) for 10 min. To terminate the transport process, the plates were chilled on ice and the cells were immediately washed with ice-cold buffer three times. Cells were lysed with 0.3 mL lysis buffer (0.5% triton x-100) and shaken vigorously for 20 min. The radioactivity of the cell lysate was counted using a MicroBeta2 Liquid Scintillation and Luminescence Counter (PerkinElmer Life Sciences). Protein concentration of the lysate was used to normalize uptake activity.
The inhibition rate was calculated using the following formula:
Inhibition Rate (%) = [(1 − (A − C)/(B − C)] × 100
A, [3H] uptake value of test compound in [3H]-TCA buffer added to pcDNA3.1/ASBT transfected cells; B, [3H] uptake value of DMSO (without inhibitor) in [3H]-TCA buffer added to pcDNA3.1/ASBT transfected cells; C, [3H] uptake value of Blank sample (DMSO) in [3H]-TCA buffer added to empty vector (pcDNA3.1) transfected cells. The IC50 value was defined as the inhibitor concentration that gives 50% taurocholate uptake compared to the no inhibitor control and calculated using SigmaPlot 10.0 software.
4. Conclusions
A series of novel arylsulfonylaminobenzanilide derivatives were designed and synthesized. Their inhibitory activities against ASBT were assessed. In general, most of them had considerable ASBT inhibitory activity. In particular, four compounds (5a8, 5g1,5g2 and 5g3) were superior to the lead compound S-1647, especially compound 5g2 which exhibited the most inhibitory effect on ASBT transport activity with an IC50 value of 0.11 μM, or 14-fold more potent than S-1647. It is not known if these compounds can pass the cell membrane using a transporter or permissively, so future studies will address this issue in vitro in cells and in vivo in animal models. It is unlikely that these compounds will have much cytotoxicity as both HEK293T and CACO2 cells showed no morphological differences when treated with 20 μM of the compounds for 24 h.
Acknowledgments
We are grateful to the financial support from the National S&T Major Special Project on Major New Drug Innovation (2012ZX093101002-001-017) and the National Natural Science Foundation of China (No. 81072577 and 81102376).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bays, H.; Stein, E.A. Pharmacotherapy for dyslipidaemia-current therapies and future agents. Expert. Opin. Pharmacother. 2003, 4, 1901–1938. [Google Scholar] [CrossRef]
- Stark, R.M. Review of the major intervention trials of lowering coronary artery disease risk through cholesterol reduction. Am. J. Cardiol. 1996, 78, 13–19. [Google Scholar] [CrossRef]
- Dawson, P.A. Role of the intestinal bile acid transporters in bile acid and drug disposition. Handb. Exp. Pharmacol. 2011, 201, 169–203. [Google Scholar] [CrossRef]
- Ast, M.; Frishman, W.H. Bile acid sequestrants. J. Clin. Phatmacol. 1990, 30, 99–106. [Google Scholar] [CrossRef]
- Knopp, R.H. Drug treatment of lipid disorders. N. Engl. J. Med. 1999, 341, 498–511. [Google Scholar] [CrossRef]
- Dietschy, J.M.; Turley, S.D.; Spady, D.K. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J. Lipid. Res. 1993, 34, 1637–1659. [Google Scholar]
- Hofmann, A.F. The continuing importance of bile acids in liver and intestinal disease. Arch. Intern. Med. 1999, 159, 2647–2658. [Google Scholar] [CrossRef]
- Shneider, B.L. Intestinal bile acid transport: Biology, physiology, and pathophysiology. J. Pediatr. Gastroenterol. Nutr. 2001, 32, 407–417. [Google Scholar] [CrossRef]
- Oelkers, P.; Kirby, L.C.; Heubi, J.E.; Dawson, P.A. Primary bile acid malabsorption casused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2). J. Clin. Invest. 1997, 99, 1880–1887. [Google Scholar] [CrossRef]
- Zhang, E.Y.; Phelps, M.A.; Cheng, C.; Ekins, S.; Swaan, P.W. Modeling of active transport systems. Adv. Drug Deliv. Rev. 2002, 54, 329–354. [Google Scholar] [CrossRef]
- Geyer, J.; Wilke, T.; Petzinger, E. The solute carrier family SLC10: More than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn. Schmiedebergs Arch. Pharmacol. 2006, 372, 413–431. [Google Scholar] [CrossRef]
- Tremont, S.J.; Lee, L.F.; Huang, H.C.; Keller, B.T.; Banerjee, S.C.; Both, S.R. Discovery of potent, nonsystemic apical sodium-codependent bile acid transporter inhibitors (Part 1). J. Med. Chem. 2005, 48, 5837–5852. [Google Scholar] [CrossRef]
- Huang, H.C.; Tremont, S.J.; Lee, L.F.; Keller, B.T.; Carpenter, A.J.; Wang, C.C.; et al. Discovery of potent, nonsystemic apical sodium-codependent bile acid transporter inhibitors (Part 2). J. Med. Chem. 2005, 48, 5853–5868. [Google Scholar] [CrossRef]
- West, K.L.; Zern, T.L.; Butteiger, D.N.; Keller, B.T.; Fernandez, M.L. SC-435, an ileal apical sodium co-dependent bile acid transporter (ASBT) inhibitor lowers plasma cholesterol and reduces atherosclerosis in guinea pigs. Atherosclerosis 2003, 171, 201–210. [Google Scholar] [CrossRef]
- Liu, X.; Chism, J.P.; Lecluyse, E.L.; Brouwer, K.R.; Brouwe, K.L. Correlation of biliary excretion in sandwich-cultured rat hepatocytes and in vivo in rats. Drug. Metab. Dispos. 1999, 27, 637–644. [Google Scholar]
- Kitayama, K.; Nakai, D.; Kone, K.; Van der Hoop, A.G.; Kurata, H.; De Wit, E.C.; Cohen, L.H.; Inaba, T.; Kohama, T. Novel non-systemic inhibitor of ileal apical Na+-dependent bile acid transporter reduces serum cholesterol levels in hamsters and monkeys. Eur. J. Pharm. 2006, 539, 89–98. [Google Scholar] [CrossRef]
- Baringhaus, K.H.; Matter, H.; Stengelin, S.; Kramer, W. Substrate specificity of the ileal and the hepatic Na+/bile acid cotransporters of the rabbit. II. A reliable 3D QSAR pharmacophore model for the ileal Na+/bile acid cotransporter. J. Lipid. Res. 1999, 40, 2158–2168. [Google Scholar]
- Lionarons, D.A.; Boyer, J.L.; Cai, S.Y. Evolution of substrate specificity for the bile salt transporter ASBT (SLC10A2). J. Lipid Res. 2012, 53, 1535–1542. [Google Scholar] [CrossRef]
- Liang, D.; Hagenbuch, B.; Stieger, B.; Meier, P.J. Parallel decrease of Na(+)-taurocholate cotransport and its encoding mRNA in primary cultures of rat hepatocytes. Hepatology. 1993, 18, 1162–1166. [Google Scholar]
- Sample Availability: Samples of the compounds 5a9 and 5a10 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).