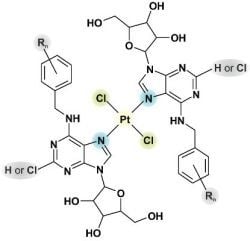
N6-Benzyladenosine Derivatives as Novel N-Donor Ligands of Platinum(II) Dichlorido Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterizations and General Properties of the Complexes
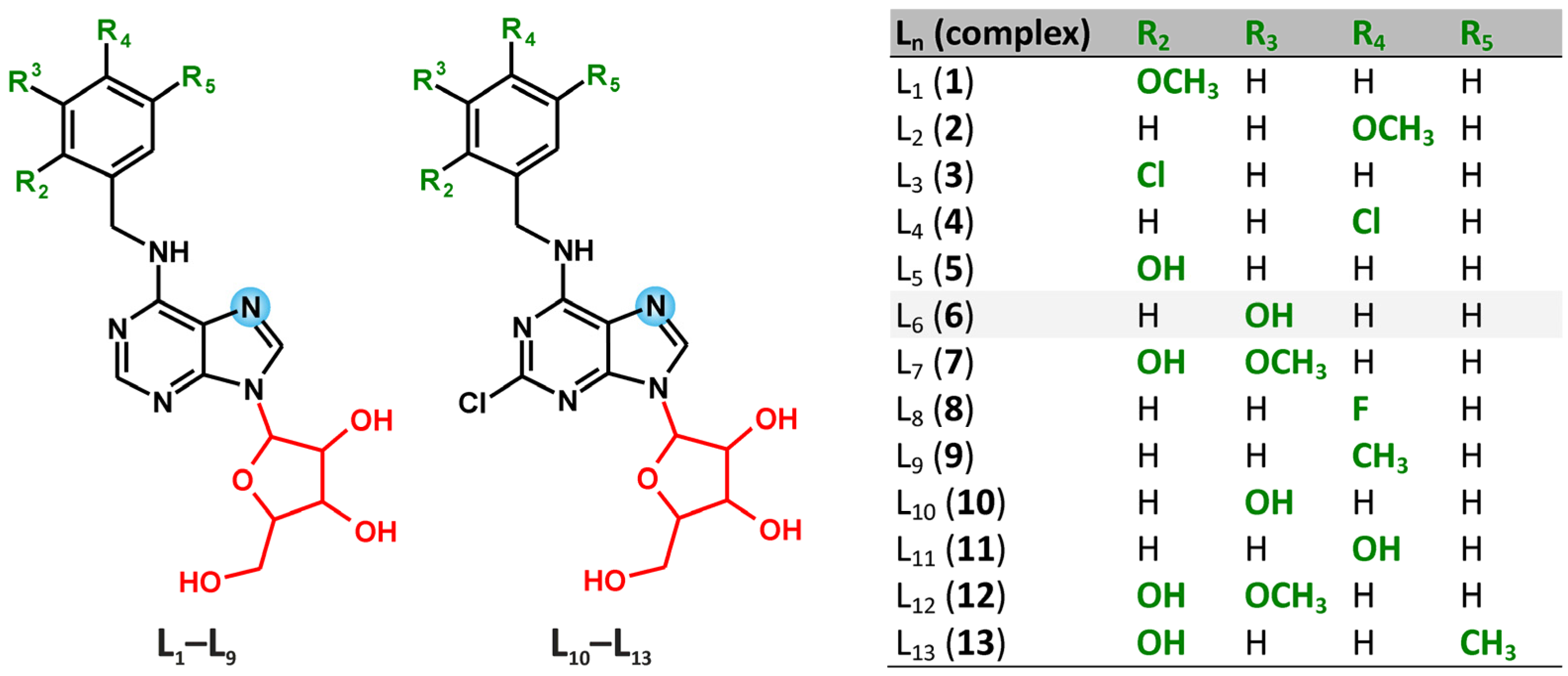
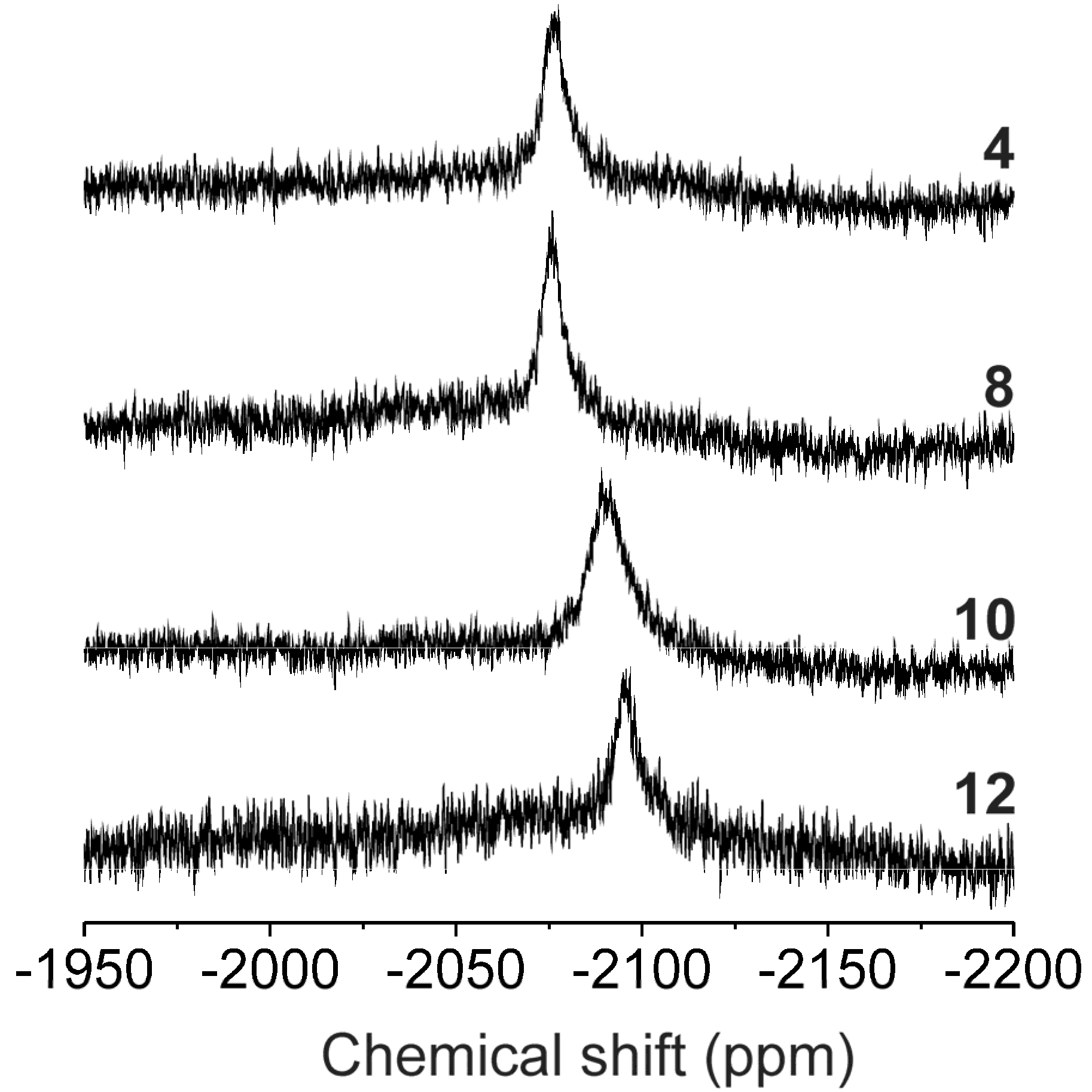
| 1H-NMR | 13C-NMR | 15N-NMR | 195Pt | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C2H | N6H | C8H | C2 | C4 | C5 | C6 | C8 | N1 | N3 | N6 | N7 | N9 | ||
| 1 | −0.09 | 0.82 | 0.84 | 1.35 | −0.82 | −4.07 | −2.36 | 2.76 | 3.82 | 1.17 | 8.47 | −105.03 | 5.60 | −2077.10 |
| 2 | 0.18 | 0.52 | 0.84 | 1.41 | −0.85 | −4.03 | −2.28 | 2.92 | 5.03 | 2.00 | 6.67 | −105.02 | 6.17 | −2072.08 |
| 3 | 0.17 | 0.60 | 0.88 | 1.37 | −0.80 | −4.01 | −2.27 | 2.96 | 3.24 | 0.82 | 6.57 | −106.00 | 4.96 | −2074.81 |
| 4 | 0.19 | 0.51 | 0.92 | 1.27 | −0.98 | −4.14 | −2.37 | 2.88 | 3.61 | 1.55 | 4.96 | −106.69 | 5.45 | −2071.38 |
| 5 | 0.12 | 0.69 | 0.72 | 1.61 | −0.82 | −3.90 | −1.91 | 2.62 | 7.11 | 0.70 | 5.05 | −105.70 | 5.09 | −2076.80 |
| 6 | 0.18 | 0.59 | 0.84 | 1.31 | −0.88 | −4.07 | −2.29 | 2.93 | 3.85 | 1.91 | 5.51 | −105.78 | 6.21 | −2074.68 |
| 7 | 0.15 | 0.75 | 0.69 | 1.49 | −0.92 | −3.97 | −4.06 | 2.73 | 6.98 | 0.34 | 5.82 | −106.22 | 4.78 | −2078.30 |
| 8 | 0.19 | 0.52 | 0.90 | 1.31 | −0.93 | −4.13 | −2.29 | 2.90 | 3.67 | 0.33 | 6.52 | −105.37 | 6.32 | −2071.68 |
| 9 | 0.19 | 0.55 | 0.83 | 1.34 | −0.88 | −4.07 | −2.32 | 2.89 | 4.46 | 1.76 | 4.89 | −106.10 | 5.86 | −2073.15 |
| 10 | − | 0.26 | 0.83 | −0.53 | −1.19 | −3.76 | −0.66 | 3.03 | 3.13 | −0.31 | 6.15 | −106.52 | 5.02 | −2084.94 |
| 11 | − | 0.60 | 0.83 | 1.30 | −1.01 | −3.68 | −2.18 | 3.14 | 3.45 | 0.26 | 3.30 | −106.24 | 5.00 | −2083.47 |
| 12 | − | 0.81 | 0.69 | 1.34 | −0.90 | −3.66 | −2.31 | 3.04 | 3.92 | 0.16 | 4.90 | −105.72 | 5.03 | −2088.28 |
| 13 | − | 0.85 | 0.78 | 0.12 | −0.83 | −3.27 | −0.60 | 1.81 | 4.89 | – | 4.96 | −105.76 | 5.33 | −2091.62 |
2.2. In Vitro Cytotoxic Activity
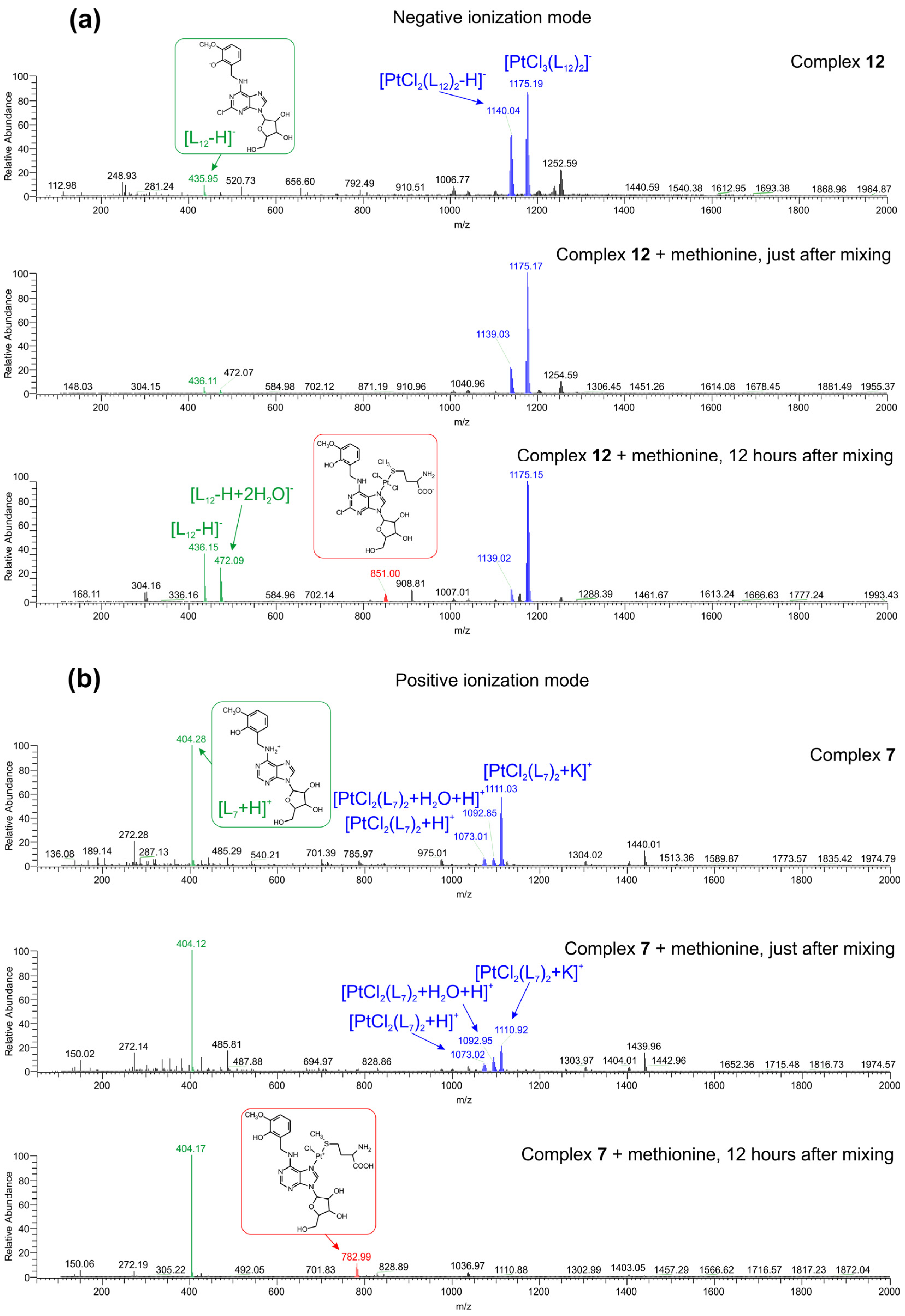
2.3. Interactions of the Selected Complexes 7 and 12 with l-Methionine, Evaluated by Mass Spectrometry
3. Experimental
3.1. Materials
3.2. Methods
3.3. Synthesis
3.4. In Vitro Cytotoxic Activity Testing
3.5. Interactions of the Selected Complexes 7 and 12 with L-Methionine, Evaluated by Mass Spectrometry
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Evaluate Ltd. EvaluatePharma® Coverage of Marketed and Pipeline Products (Platinum Compounds). Available online: http://www.evaluategroup.com/Universal/View.aspx?type =Entity&entityType=Product&lType=modData&id=438&componentID=1003/ (accessed on 24 April 2013).
- Kauffmann, G.B.; Pentimalli, R.; Doldi, S.; Hall, M.D. Michele Peyrone (1813–1883), Discoverer of Cisplatin. Platinum Metals Rev. 2010, 54, 250–256. [Google Scholar] [CrossRef]
- Rosenberg, B.; VanCamp, L.; Trosko, J.E.; Mansour, V.H. Platinum compounds: A new class of potent antitumour agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Perez, J.M.; Fuertes, M.A.; Alonso, C.; Navarro-Ranninger, C. Current status of the development of trans-platinum antitumor drugs. Crit. Rev. Oncol./Hematol. 2000, 35, 109–120. [Google Scholar] [CrossRef]
- Kalinowska-Lis, U.; Ochocki, J.; Matlawska-Wasowska, K. Trans geometry in platinum antitumor complexes. Coord. Chem. Rev. 2008, 252, 1328–1345. [Google Scholar] [CrossRef]
- Farrell, N.; Ha, T.T.B.; Souchard, J.P.; Wimmer, F.L.; Cros, S.; Johnson, N.P. Cytostatic trans-platinum(II) complexes. J. Med. Chem. 1989, 32, 2240–2241. [Google Scholar] [CrossRef]
- Montero, E.I.; Díaz, S.; González-Vadillo, A.M.; Pérez, J.M.; Alonso, C.; Navarro-Ranninger, C. Preparation and characterization of novel trans-[PtCl 2(amine)(isopropylamine)] compounds. Cytotoxic activity and apoptosis induction in ras-transformed cells. J. Med. Chem. 1999, 42, 4264–4268. [Google Scholar] [CrossRef]
- Bocarelli, A.; Coluccia, M.; Intini, F.P.; Natile, G.; Locker, D.; Leng, M. Cytotoxicity and DNA binding mode of new platinum-iminoether derivatives with different configuration of the iminoether ligands. Anti-Cancer Drug Des. 1999, 14, 253–264. [Google Scholar]
- Coluccia, M.; Nassi, A.; Bocarelli, A.; Giordano, D.; Cardellicchio, N.; Locker, D.; Leng, M.; Sivo, M.; Intini, F.P.; Natile, G. In vitro and in vivo antitumor activity of and cellular pharmacological properties of new platinum-iminoether complexes with different configuration of the iminoether ligands. J. Inorg. Biochem. 1999, 77, 31–35. [Google Scholar] [CrossRef]
- Perego, P.; Caserini, C.; Gatti, L.; Carenni, N.; Romanelli, S.; Supino, R.; Colangelo, D.; Viano, Y.; Leone, R.; Spinelli, S.; et al. A novel trinuclear platinum complex overcomes cisplatin resistance in an osteosarcoma cell system. Mol. Pharm. 1999, 55, 528–534. [Google Scholar]
- Skoog, F.; Hamzi, H.G.; Szweykowska, A.M.; Leonard, N.J.; Carraway, K.L.; Fujii, T.; Helgeson, J.P.; Loeppky, R.N. Cytokinins: Structure/activity relationships. Phytochemistry 1967, 6, 1169–1192. [Google Scholar]
- Doležal, K.; Popa, I.; Zatloukal, M.; Lenobel, R.; Hradecká, D.; Vojtěšek, B.; Uldrijan, S.; Mlejnek, P.; Werbrouck, S.; Strnad, M. Substitution derivatives of N6-benzyladenosine, methods of their preparation, their use for preparation of drugs, cosmetic preparations and growth regulators, pharmaceutical preparations, cosmetic preparations and growth regulators containing these compounds. U.S. Patent 8,119,614 B2, 21 February 2012. [Google Scholar]
- Doležel, P.; Koudelková, P.; Mlejnek, P. Halogenation of N6-benzyladenosine decreases its cytotoxicity in human leukemia cells. Toxicol. In Vitro 2010, 24, 2079–2083. [Google Scholar] [CrossRef]
- Waysbort, D.; Tarien, E.; Eichhorn, G.L. Nature of the specific interaction of rhodium acetate dimer with adenosine. Inorg. Chem. 1993, 32, 4774–4779. [Google Scholar] [CrossRef]
- Trávníček, Z.; Mikulík, J.; Čajan, M.; Zbořil, R.; Popa, I. Novel iron complexes bearing N6-substituted adenosine derivatives: Synthesis, magnetic, 57Fe Mossbauer, DFT, and in vitro cytotoxicity studies. Bioorg. Med. Chem. 2008, 16, 8719–8728. [Google Scholar] [CrossRef]
- Szüčová, L.; Trávníček, Z.; Popa, I.; Marek, J. Preparation and cis-to-trans transformation study of square-planar [Pt(Ln)2Cl2] complexes bearing cytokinins derived from 6-benzylaminopurine (Ln) by view of NMR spectroscopy and X-ray crystallography. Polyhedron 2008, 27, 2710–2720. [Google Scholar] [CrossRef]
- Štarha, P.; Trávníček, Z.; Popa, A.; Popa, I.; Muchová, T.; Brabec, V. How to modify 7-azaindole to form cytotoxic Pt(II) complexes: Highly in vitro anticancer effective cisplatin derivatives involving halogeno-substituted 7-azaindole. J. Inorg. Biochem. 2012, 115, 57–63. [Google Scholar] [CrossRef]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Pouchert, C.J. The Aldrich Library of Infrared Spectra; Aldrich Chemical Company Press: Milwaukee, WI, USA, 1981. [Google Scholar]
- Čajan, M.; Trávníček, Z. Structural (X-ray), spectral (FT-IR and Raman) and quantum chemical investigations of a series of 6-benzylaminopurine derivatives. J. Mol. Struct. 2011, 994, 350–359. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. Part B: Applications in Coordination, Organometallic and Bioinorganic Chemistry; John Wiley & Sons Ltd.: New York, NY, USA, 1991. [Google Scholar]
- Terzis, A.; Hadjiliadis, N.; Rivest, R.; Theophanides, T. X-ray crystal structure of a platinum-9-methyladenine complex. Inorg. Chim. Acta 1975, 12, L5–L6. [Google Scholar] [CrossRef]
- Wexselblatt, E.; Gibson, D. What do we know about the reduction of Pt(IV) pro-drugs? J. Inorg. Biochem. 2012, 117, 220–229. [Google Scholar] [CrossRef]
- Sanchez-Canoa, C.; Hannon, M.J. Novel and emerging approaches for the delivery of metallo-drugs. Dalton Trans. 2009, 10702–10711. [Google Scholar] [CrossRef]
- Chung, J.S.; Haque, R.; Guha Mazumder, D.N.; Moore, L.E.; Ghosh, N.; Samanta, S.; Mitra, S.; Hira-Smith, M.M.; von Ehrenstein, O.; Basu, A.; et al. Blood concentrations of methionine, selenium, beta-carotene, and other micronutrients in a case-control study of arsenic-induced skin lesions in West Bengal, India. Environ. Res. 2006, 101, 230–237. [Google Scholar] [CrossRef]
- Videhult, P.; Laurell, G.; Wallin, I.; Ehrsson, H. Kinetics of cisplatin and its monohydrated complex with sulfur-containing compounds designed for local otoprotective administration. Exp. Biol. Med. 2006, 231, 1638–1645. [Google Scholar]
- Farrell, N.; Kelland, L.R.; Roberts, J.D.; Van Beusichem, M. Activation of the trans geometry in platinum antitumor complexes: a survey of the cytotoxicity of trans complexes containing planar ligands in murine L1210 and humar tumor panels and studies on their mechanism of action. Cancer Res. 1992, 52, 5065–5072. [Google Scholar]
- Kuhnle, J.A.; Fuller, G.; Corse, J.; Mackey, B.E. Anti-senescent activity of natural cytokinins. Physiol. Plantarum 1977, 41, 14–21. [Google Scholar] [CrossRef]
- Novikov, A.V.; Bublyaev, R.A.; Krasnov, N.V.; Kozmin, Y.P.; Mirgorodskaya, O.A. ESI-MS studies of silver ion competitive interaction with cysteine-containing peptides and sulfur-containing amino acids. Protein Pept. Lett. 2010, 17, 1392–1397. [Google Scholar] [CrossRef]
- Canon, F.; Paté, F.; Meudec, E.; Marlin, T.; Cheynier, V.; Giuliani, A.; Sarni-Manchado, P. Characterization, stoichiometry, and stability of salivary protein-tannin complexes by ESI-MS and ESI-MS/MS. Anal. Bioanal. Chem. 2009, 395, 2535–2545. [Google Scholar]
- Sandercock, A.; Robinson, C. Electrospray Ionization Mass Spectrometry and the Study of Protein Complexes. In Protein Interactions; Schuck, P., Ed.; Springer: New York, NY, USA, 2007; Volume 1, pp. 447–468. [Google Scholar]
- Sample Availability: Samples of the compounds 1–13 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Štarha, P.; Popa, I.; Trávníček, Z.; Vančo, J. N6-Benzyladenosine Derivatives as Novel N-Donor Ligands of Platinum(II) Dichlorido Complexes. Molecules 2013, 18, 6990-7003. https://doi.org/10.3390/molecules18066990
Štarha P, Popa I, Trávníček Z, Vančo J. N6-Benzyladenosine Derivatives as Novel N-Donor Ligands of Platinum(II) Dichlorido Complexes. Molecules. 2013; 18(6):6990-7003. https://doi.org/10.3390/molecules18066990
Chicago/Turabian StyleŠtarha, Pavel, Igor Popa, Zdeněk Trávníček, and Ján Vančo. 2013. "N6-Benzyladenosine Derivatives as Novel N-Donor Ligands of Platinum(II) Dichlorido Complexes" Molecules 18, no. 6: 6990-7003. https://doi.org/10.3390/molecules18066990
APA StyleŠtarha, P., Popa, I., Trávníček, Z., & Vančo, J. (2013). N6-Benzyladenosine Derivatives as Novel N-Donor Ligands of Platinum(II) Dichlorido Complexes. Molecules, 18(6), 6990-7003. https://doi.org/10.3390/molecules18066990