Studies on the Alkaloids of the Bark of Magnolia officinalis: Isolation and On-line Analysis by HPLC-ESI-MSn
Abstract
:1. Introduction
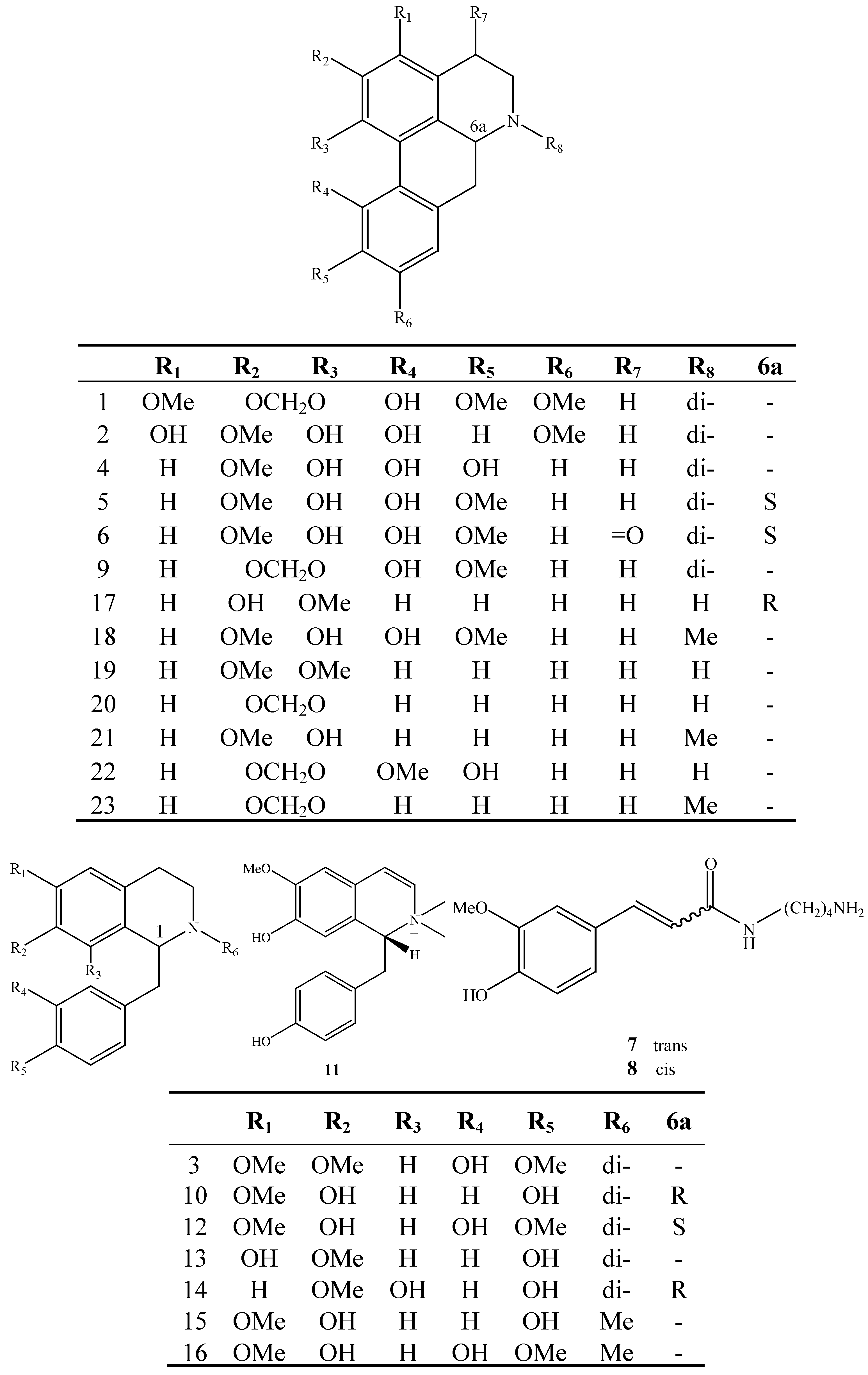
2. Results and Discussion
2.1. Structure Elucidation of the Purified Compounds
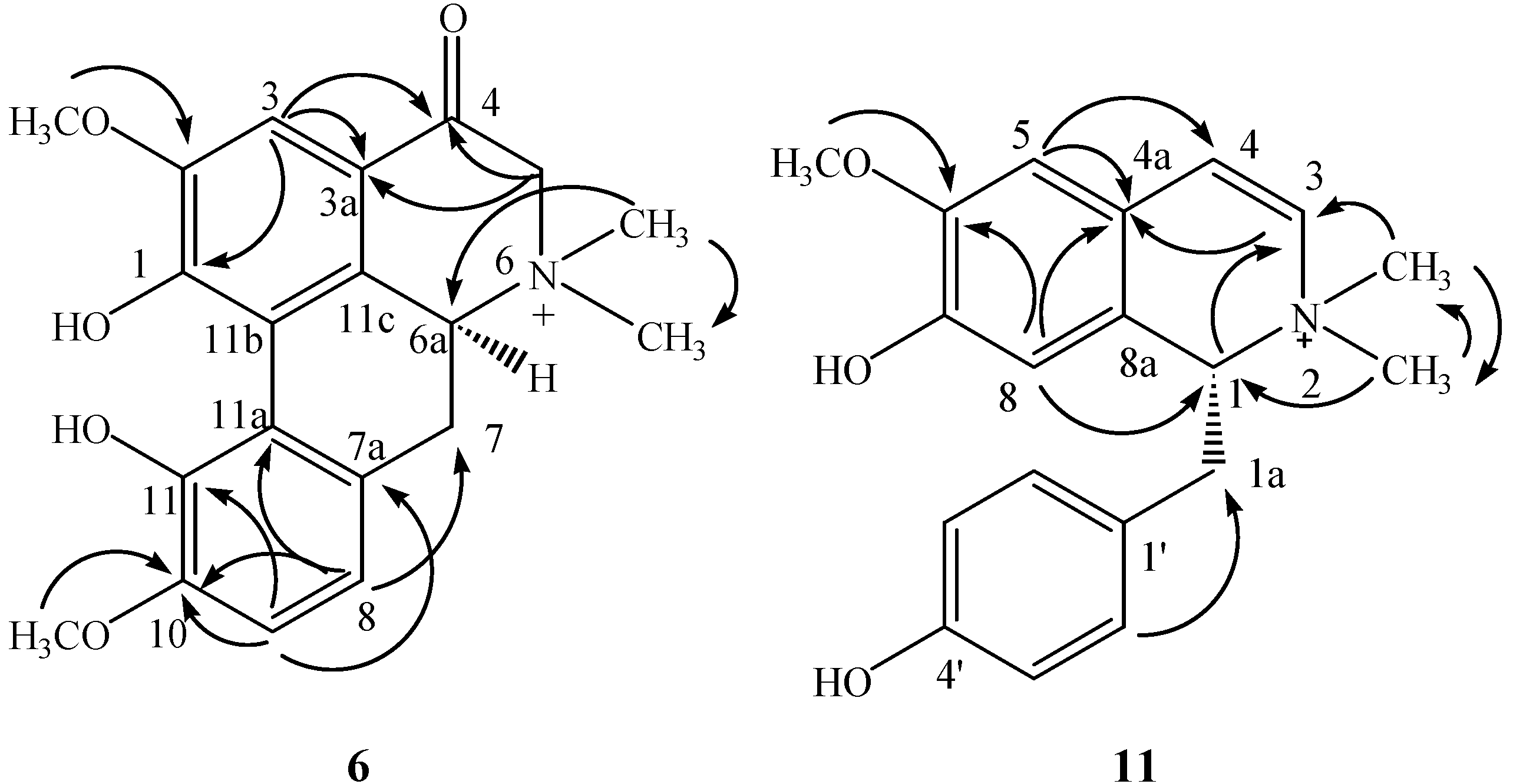
2.2. Bioactivity
2.3. HPLC-ESI-MSn Analysis of Alkaloids in M. officinalis
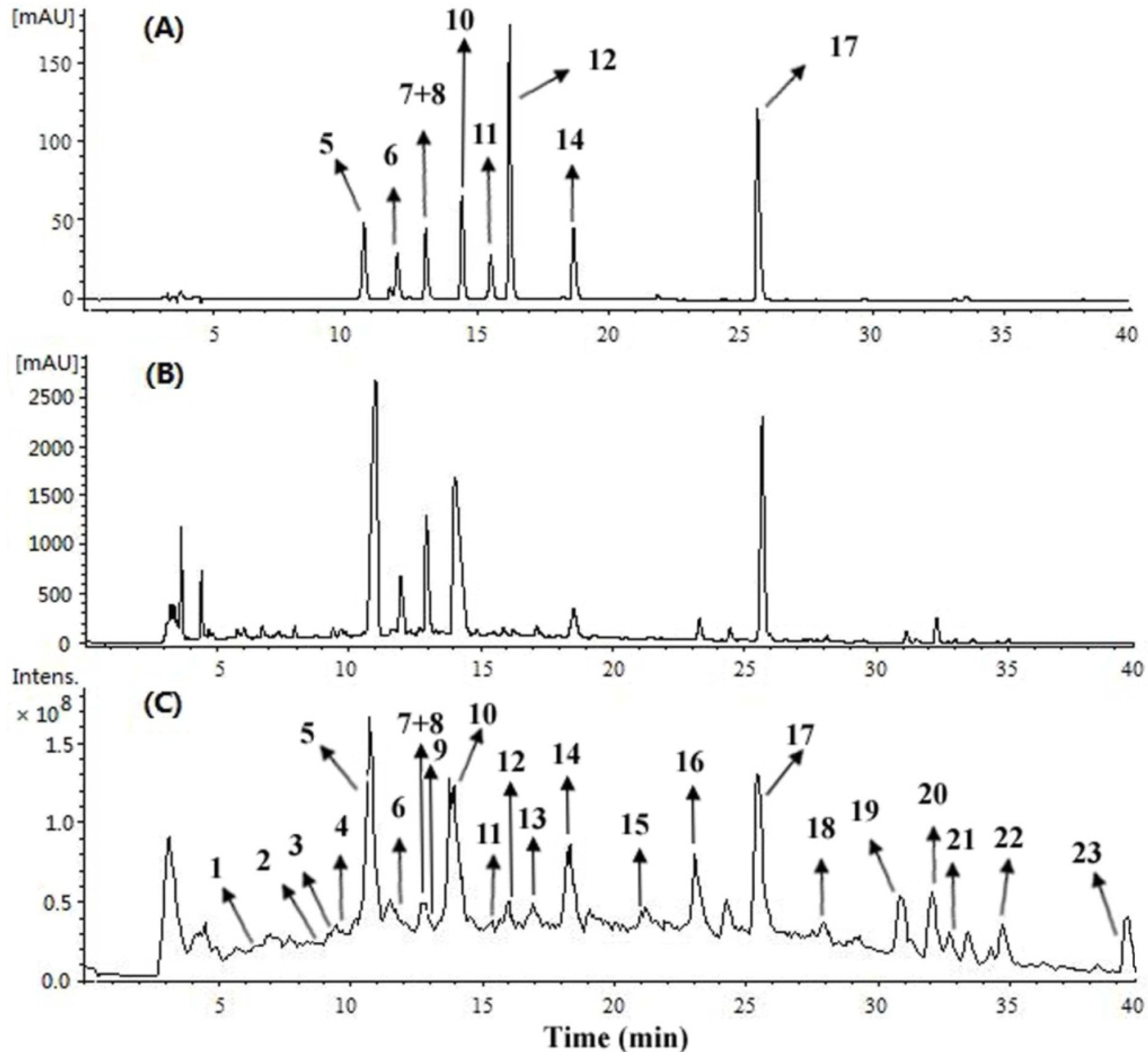
| Peak no. | tR (min) | Proposed compounds | MS | ESI-MSn m/z (% base peak) |
|---|---|---|---|---|
| 1 | 6.3 | N-Methylocoxylonine (1) | 400 [M]+ | MS2 [400]: 369 (10), 355 (100), 337 (15), 323 (95); MS3 [355]: 340 (5), 323 (100), 309 (7); MS3 [323]: 295 (100) |
| 2 | 8.7 | 1,3,11-Trihydroxy-2,9-dimethoxy-6,6-dimethylaporphinium (2) | 358 [M]+ | MS2 [358]: 340 (20), 313 (100), 295 (80), 281 (75); MS3 [313]: 298 (20), 285 (65), 281(90), 253 (100) |
| 3 | 9.3 | N-Methyllaudanidinium (3) | 358 [M]+ | MS2 [358]: 340 (11), 313 (100), 295 (60), 221 (54); MS3 [313]: 295 (20), 285 (99), 281 (100), 253 (95) |
| 4 | 9.9 | 1,9,10-Trihydroxy-2-methoxy-6,6-dimethylaporphinium (4) | 328 [M]+ | MS2 [328]: 283 (98), 265(90), 257 (100) |
| 5 | 10.8 | (S)-Magnoflorine (5) | 342 [M]+ | MS2 [342]: 311 (20), 297 (100), 265 (53); MS3 [297]: 282 (13), 264 (100), 237 (12) |
| 6 | 11.8 | (S)-4-Keto-magnoflorine (6) | 356 [M]+ | MS2 [356]: 311.0 (5), 285 (100); MS3 [285]: 270 (60), 257 (83), 253 (80), 239 (78), 221 (100) |
| 7/8 | 12.8 | trans/cis N-Feruloylputrescine (7/8) | 265 [M+H]+ | MS2 [265]: 248 (75), 177 (100); MS3 [177]: 145 (100) |
| 9 | 12.9 | N-Methylbulbocapnine (9) | 340 [M]+ | MS2 [340]: 309(5), 295 (100), 203 (17); MS3 [295]: 263 (100) |
| 10 | 14.0 | (R)-Magnocurarine (10) | 314 [M]+ | MS2 [314]: 269 (100), 175 (39), 107 (66); MS3 [269]: 237 (24), 175 (55), 107 (100) |
| 11 | 15.5 | (R)-3,4-Dehydromagnocurarine (11) | 312 [M]+ | MS2 [312]: 205 (100), 190 (20), 145 (11); MS3 [205]: 190.0 (100), 145 (15); |
| 12 | 16.2 | (S)-Tembetarine (12) | 344 [M]+ | MS2 [344]: 299 (99), 175 (100), 137 (83); MS3 [175]: 143 (100) |
| 13 | 16.9 | Lotusine (13) | 314 [M]+ | MS2 [314]: 269 (23), 237 (27), 205 (35), 175 (48),107 (100); MS3 [269]: 175 (99), 107 (100) |
| 14 | 18.4 | (R)-Oblongine (14) | 314 [M]+ | MS2 [314]: 269 (51), 254 (8), 237 (11), 175 (13), 137 (32), 107 (100) |
| 15 | 21.7 | N-Methylcoclaurine (15) | 300 [M+H]+ | MS2 [300]: 269 (100), 237 (10), 192 (15), 107 (50); MS3 [269]: 237 (20), 175 (70), 107 (100) |
| 16 | 23.3 | Reticuline (16) | 330 [M+H]+ | MS2 [330]: 299 (5), 192 (100), 177 (3), 137 (4) |
| 17 | 25.4 | (R)-Asimilobine (17) | 268 [M+H]+ | MS2 [268]: 251 (100), 219 (29); MS3 [251]: 236 (5), 219 (100), 191 (10) |
| 18 | 28.0 | Corytuberine (18) | 328 [M+H]+ | MS2 [328]: 297 (100), 265 (55); MS3 [297]: 265 (100); MS3 [265]: 237 (35), 233 (100) |
| 19 | 31.0 | Nornuciferine (19) | 282 [M+H]+ | MS2 [282]: 265 (100), 250 (5); MS3 [265]: 250 (100), 234 (21) |
| 20 | 32.1 | Anonaine (20) | 266 [M+H]+ | MS2 [266]: 249 (100); MS2 [249]: 219 (100), 191 (30); MS3 [219]: 191 (100) |
| 21 | 32.9 | Lirinidine (21) | 282 [M+H]+ | MS2 [282]: 251 (95), 219 (100), 191 (5); MS2 [251]: 219 (100), 191 (30); MS3 [219]: 191 (100) |
| 22 | 34.5 | Nandigerine (22) | 312 [M+H]+ | MS2 [312]: 295 (100); MS3 [295]: 280 (100), 265 (10), 264 (95), 263 (30) |
| 23 | 39.4 | Roemerine (23) | 280 [M+H]+ | MS2 [280]: 249 (100); MS3 [249]: 219 (100), 191 (50) |

3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Assessment of Aldose Reductase, Lipase, α-Glucosidase Inhibitory Activities
3.5. Assessment of DPP–IV Inhibitory Activity
3.6. Assessment of Cytoxic Activity
3.7. Sample Preparation
3.8. Chromatographic and MS Conditions
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Jiangsu New Medical College. Chinese Drug Dictionary; Shanghai People Publishing House: Shanghai, China, 1977; pp. 1628–1630. [Google Scholar]
- National Commission of Chinese Pharmacopoeia. Chinese Pharmacopoeia I; Chemical Industry Press: Beijing, China, 2010; p. 235. [Google Scholar]
- Sarker, S.D.; Latif, Z.; Stewart, M.; Nabar, L. Phytochemistry of the genus Magnolia. In The genusMagnolia; Sarker, S.D., Maruyama, Y., Eds.; CRC Press: Boca Raton, CA, USA, 2002; pp. 32–85. [Google Scholar]
- Cui, J.F.; Zhang, G.D.; Song, W.Z. Reversed phase ion-pair HPLC determination of quaternary ammonium alkaloids in the traditional Chinese drug Hou-po (Magnolia officinalis). Acta Pharmacol. Sin. 1988, 23, 383–387. [Google Scholar]
- Moriyasu, M.; Wang, J.; Zhang, H.; Lu, G.B.; Ichimaru, M.; Kato, A. Isolation of alkaloids from plant materials by combination of ion-pair extraction and preparative ion-pair HPLC using sodium perchlorate. III1) Chinese Magnoliae cortex. Nat. Med. 1996, 50, 413–416. [Google Scholar]
- Guo, Z.F.; Wang, X.B.; Luo, J.G.; Luo, J.; Wang, J.S.; Kong, L.Y. A novel aporphine alkaloid from Magnolia officinalis. Fitoterapia 2011, 82, 637–641. [Google Scholar]
- Yu, S.X.; Yan, R.Y.; Liang, R.X.; Wang, W.; Yang, B. Bioactive polar compounds from stem bark of Magnolia officinalis. Fitoterapia 2012, 83, 356–361. [Google Scholar] [CrossRef]
- Ishii, H.; Imai, M.; Johji, S.; Tan, S.; Chen, I.S.; Ishikawa, T. Studies on the chemical constituents of Xanthoxylum nitidum (Roxb.) D.C. (Fagara nitida Roxb.).II. Examination of the chemical constituents by membrane filtration: Identification of magnoflorine, a water-soluble quaternary aporphine alkaloid. Chem. Pharm. Bull. 1994, 42, 108–111. [Google Scholar] [CrossRef]
- Ringdahl, B.; Chan, R.P.K.; Craig, J.C.; Cava, M.P.; Shamma, M. Circular dichroism of aporphines. J. Nat. Prod. 1981, 44, 80–85. [Google Scholar] [CrossRef]
- Matsushige, A.; Kotake, Y.; Matsunami, K.; Otsuka, H.; Ohta, S.; Takeda, Y. Annonamine, a new aporphine alkaloid from the leaves of Annona muricata. Chem. Pharm. Bull. 2012, 60, 257–259. [Google Scholar] [CrossRef]
- Malmberg, A. N-Feruloylputrescine in infected Potato Tubers. Acta Chem. Scand. Ser. B 1984, 38, 153–155. [Google Scholar] [CrossRef]
- Lu, Z.M.; Zhang, Q.J.; Chen, R.Y.; Yu, D.Q. Study on chemical constituents from branches and leaves of Polyalthia nemoralis. Chin. J. Chin. MateriaMed. 2011, 36, 1024–1027. [Google Scholar]
- Wang, E.J.; Ma, Y.B.; Zhang, X.M.; Jiang, Z.Y.; Chen, J.J. Five alkaloids from vine stems of Diploclisia affinis. Chin. J. Chin. Materia Med. 2008, 33, 2503–2505. [Google Scholar]
- Liu, Q.X.; Qiu, S.Y.; Yu, H.; Ke, Y.X.; Jin, Y.; Liang, X.M. Selective separation of structure-related alkaloids in Rhizoma coptidis with “click” binaphthyl stationary phase and their structural elucidation with liquid chromatography-mass spectrometry. Analyst 2011, 136, 4357–4365. [Google Scholar]
- Hu, Y.M.; Su, G.H.; Sze, S.C.; Ye, W.C.; Tong, Y. Quality assessment of Cortex Phellodendri by high-performance liquid chromatography coupled with electrospray ionization mass spectrometry. Biomed. Chromatogra. 2010, 24, 438–453. [Google Scholar]
- Li, S.; Cui, B.S.; Liu, Q.; Tang, L.; Yang, Y.C.; Jin, X.J.; Shen, Z.F. New triterpenoids from the leaves of Cyclocarya paliurus. Planta Med. 2012, 78, 290–296. [Google Scholar]
- Hu, C.X.; Huang, H.; Zhang, L.; Huang, Y.; Shen, Z.F.; Cheng, K.D.; Du, G.H.; Zhu, P. A new screening method based on yeast-expressed human dipeptidyl peptidase IV and discovery of novel inhibitors. Biotechnol. Lett. 2009, 31, 979–984. [Google Scholar] [CrossRef]
- Zhang, P.C.; Wang, S.; Wu, Y.; Chen, R.Y.; Yu, D.Q. Five new diprenylated flavonols from the leaves of Broussonetia kazinoki. J. Nat. Prod. 2001, 64, 1206–1209. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 5–8, 10–12, 14, and 17 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yan, R.; Wang, W.; Guo, J.; Liu, H.; Zhang, J.; Yang, B. Studies on the Alkaloids of the Bark of Magnolia officinalis: Isolation and On-line Analysis by HPLC-ESI-MSn. Molecules 2013, 18, 7739-7750. https://doi.org/10.3390/molecules18077739
Yan R, Wang W, Guo J, Liu H, Zhang J, Yang B. Studies on the Alkaloids of the Bark of Magnolia officinalis: Isolation and On-line Analysis by HPLC-ESI-MSn. Molecules. 2013; 18(7):7739-7750. https://doi.org/10.3390/molecules18077739
Chicago/Turabian StyleYan, Renyi, Weihao Wang, Jian Guo, Hongliang Liu, Jianyong Zhang, and Bin Yang. 2013. "Studies on the Alkaloids of the Bark of Magnolia officinalis: Isolation and On-line Analysis by HPLC-ESI-MSn" Molecules 18, no. 7: 7739-7750. https://doi.org/10.3390/molecules18077739




