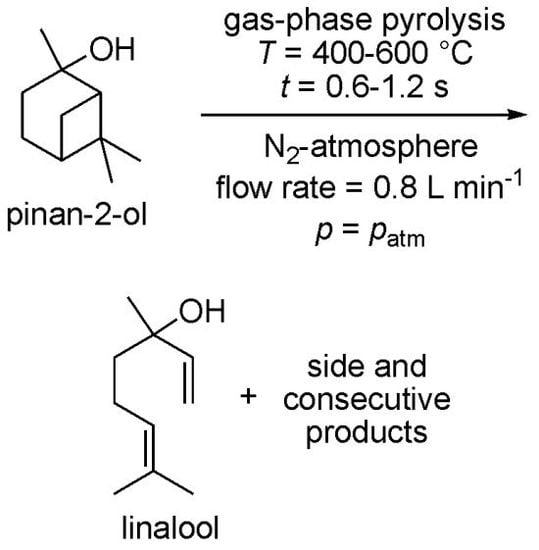
Thermal Behavior of Pinan-2-ol and Linalool
Abstract
:1. Introduction
2. Results and Discussion
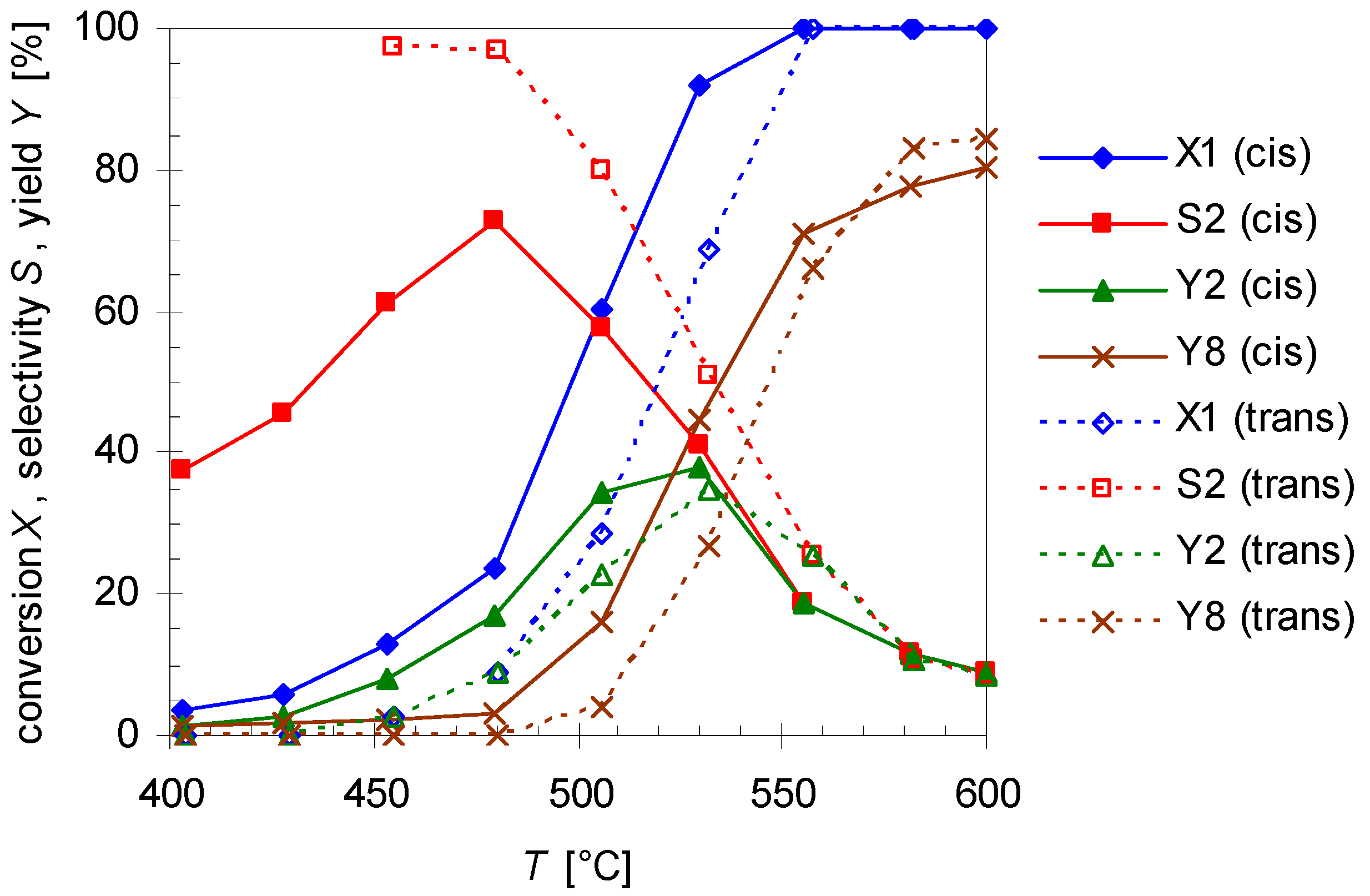
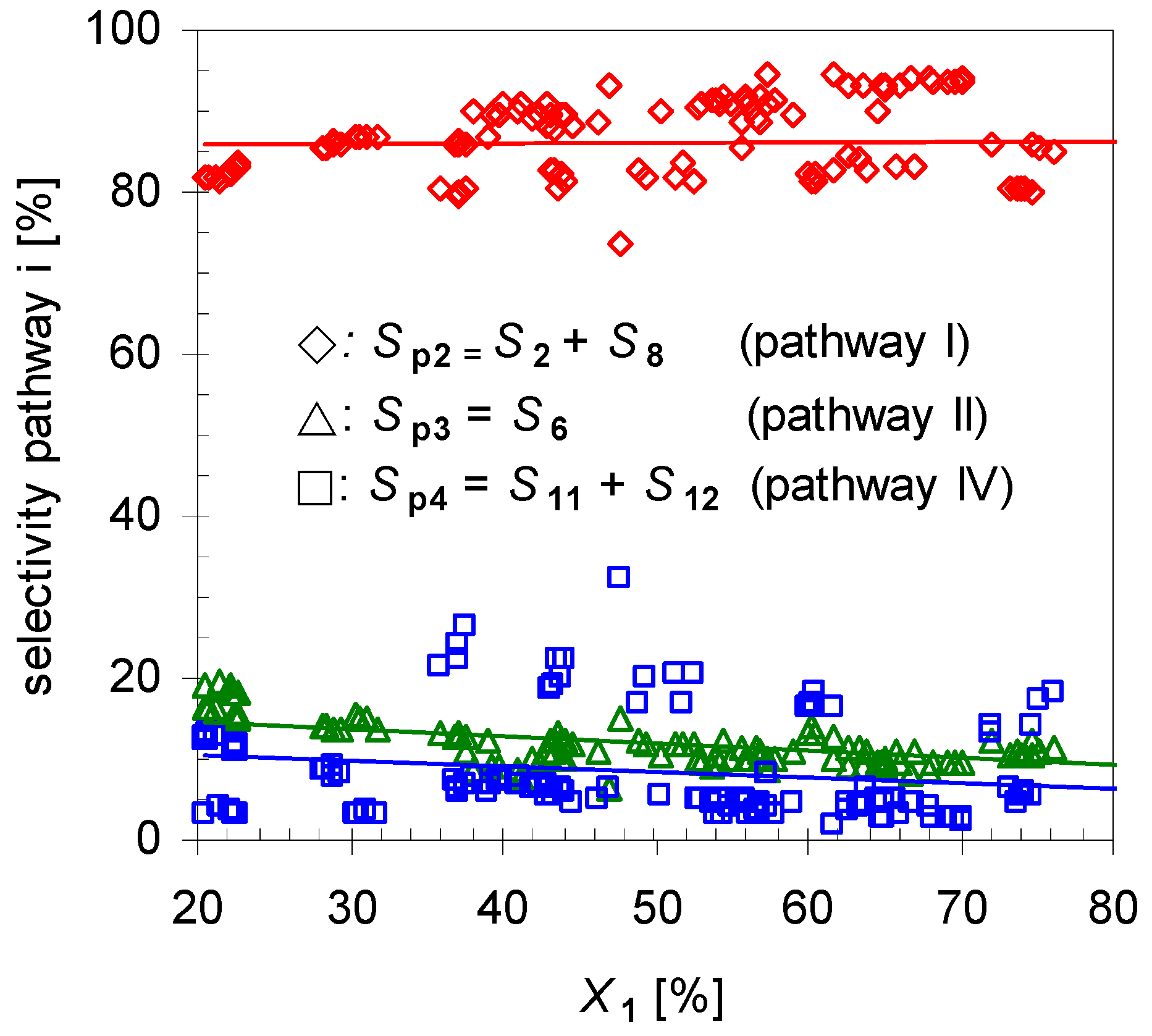
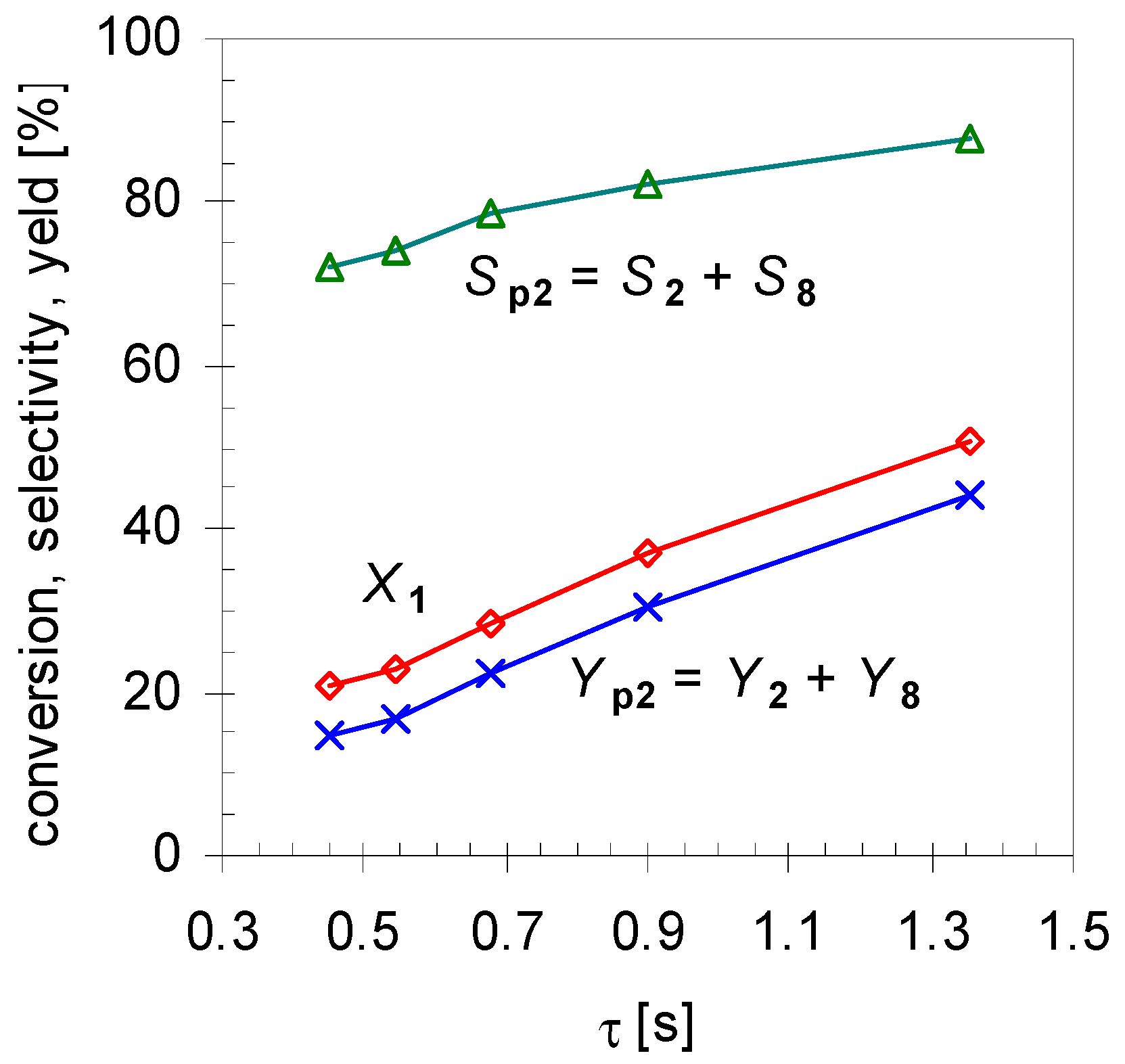
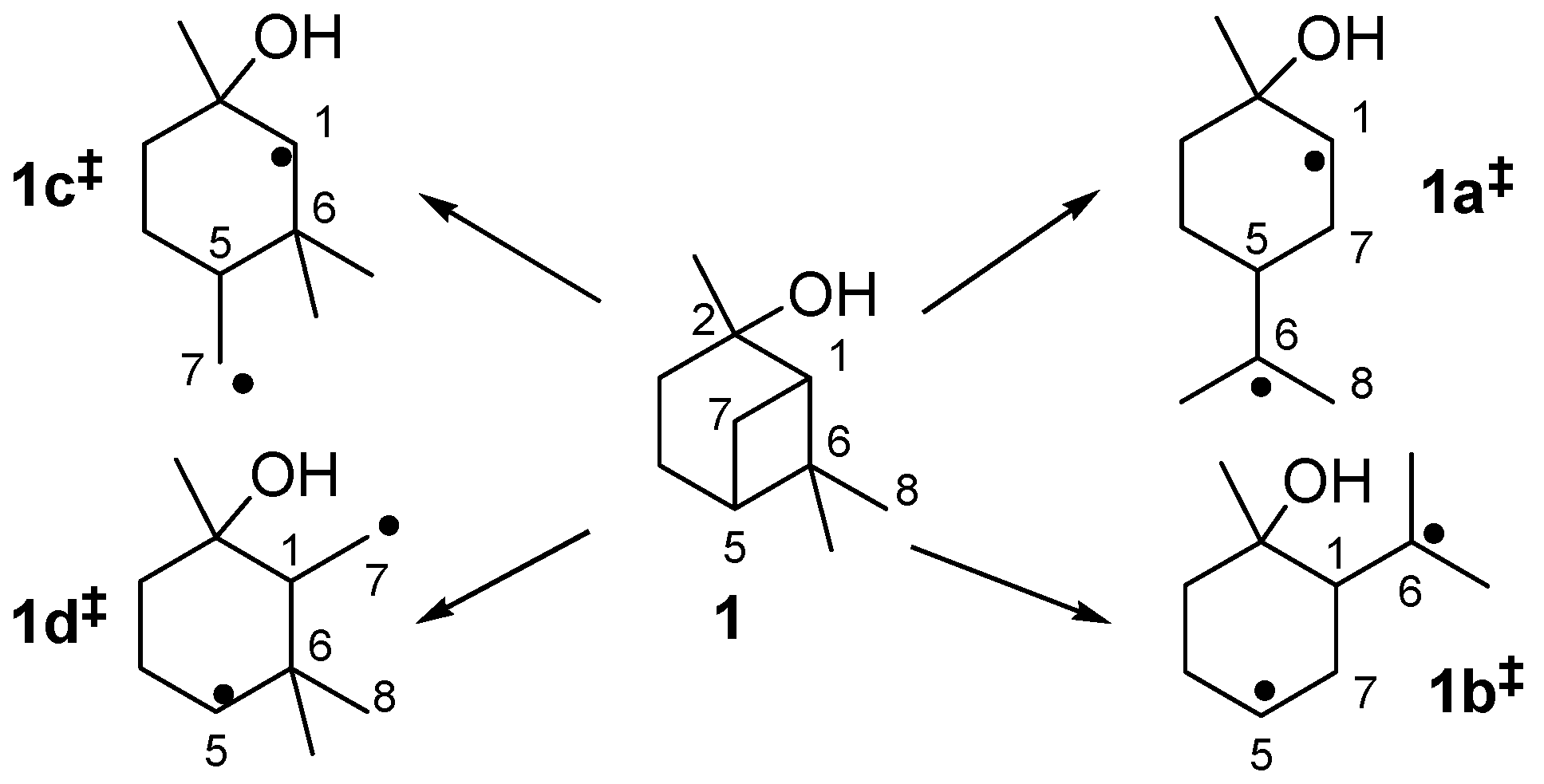
2.1. Thermal Behavior of Pinan-2-ol
- i)
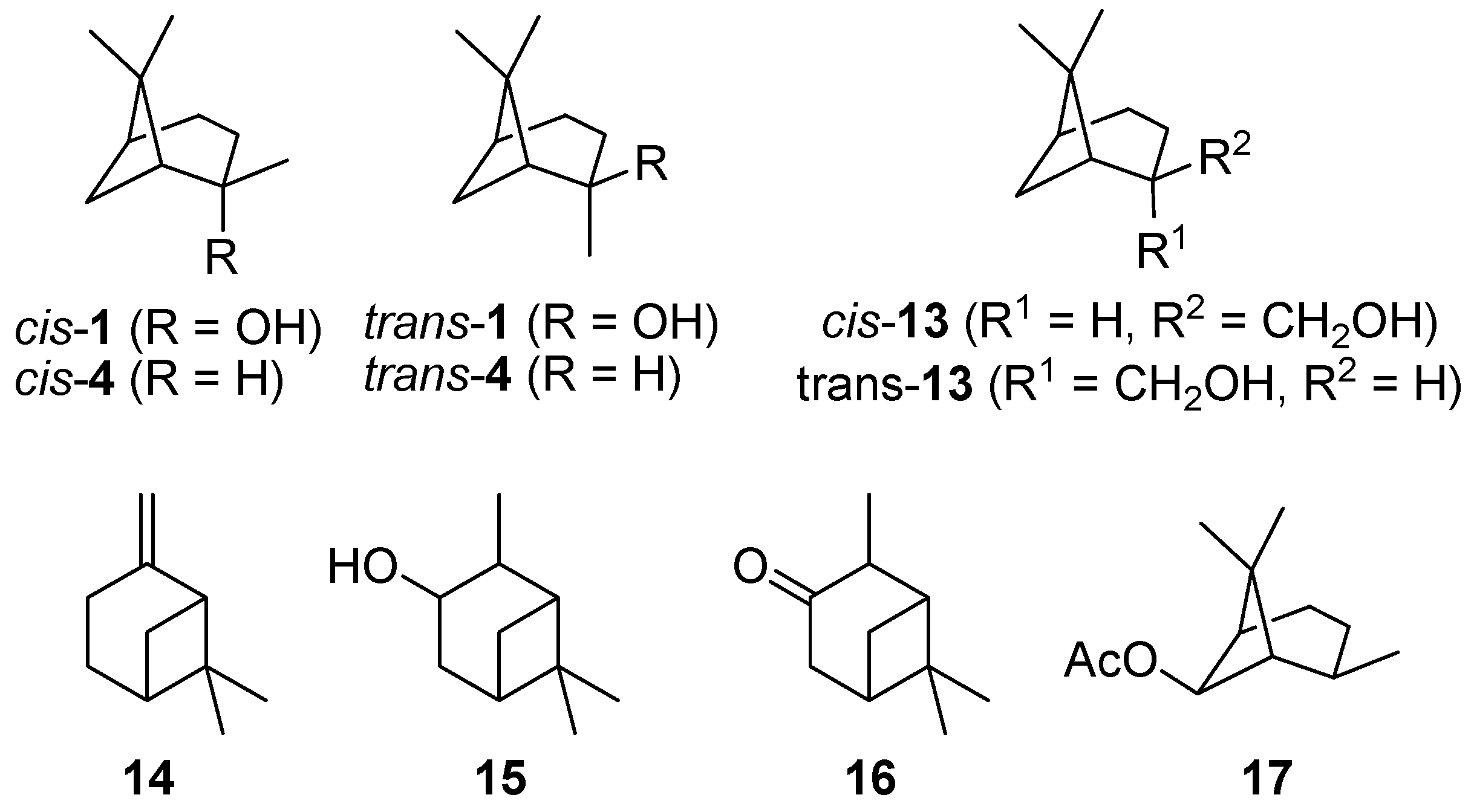
- Pathway I (index p2) leads to 2 and 8.
- ii)
- Pathway II (index p3) affords cyclohexanol 6.
- iii)
- Pathway IV (index p4) summarizes dehydration products 11 and 12.
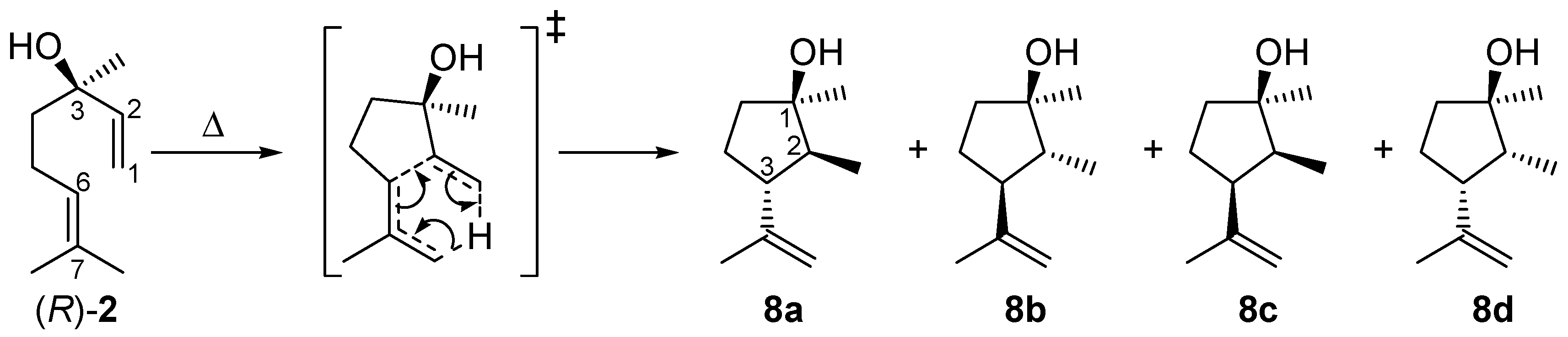
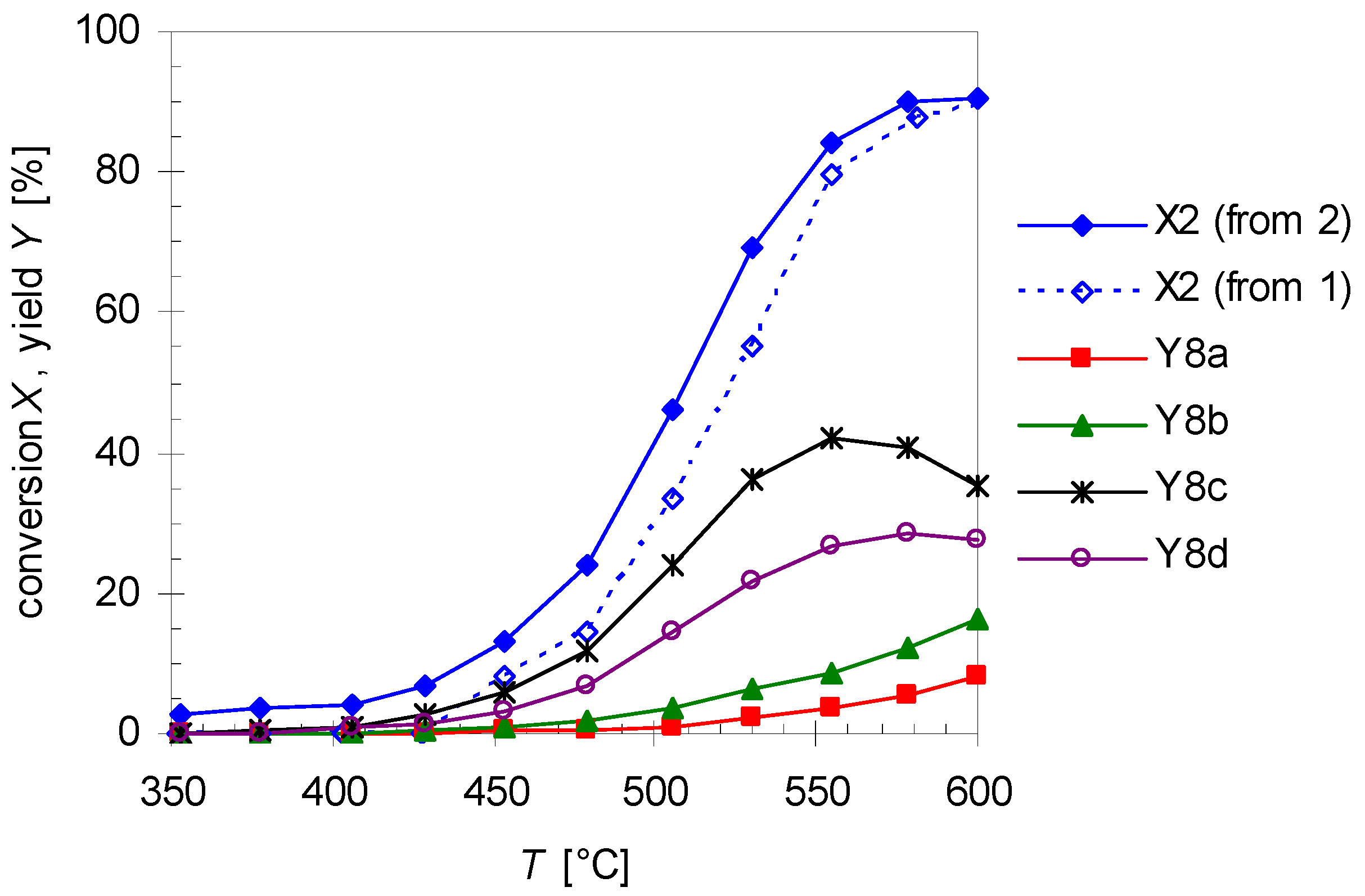
2.2. Thermal Behavior of Linalool
2.3. Mechanism of the Rearrangement of Pinan-2-ol into Linalool
2.4. Mechanism for the Rearrangement of Linalool to Plinols
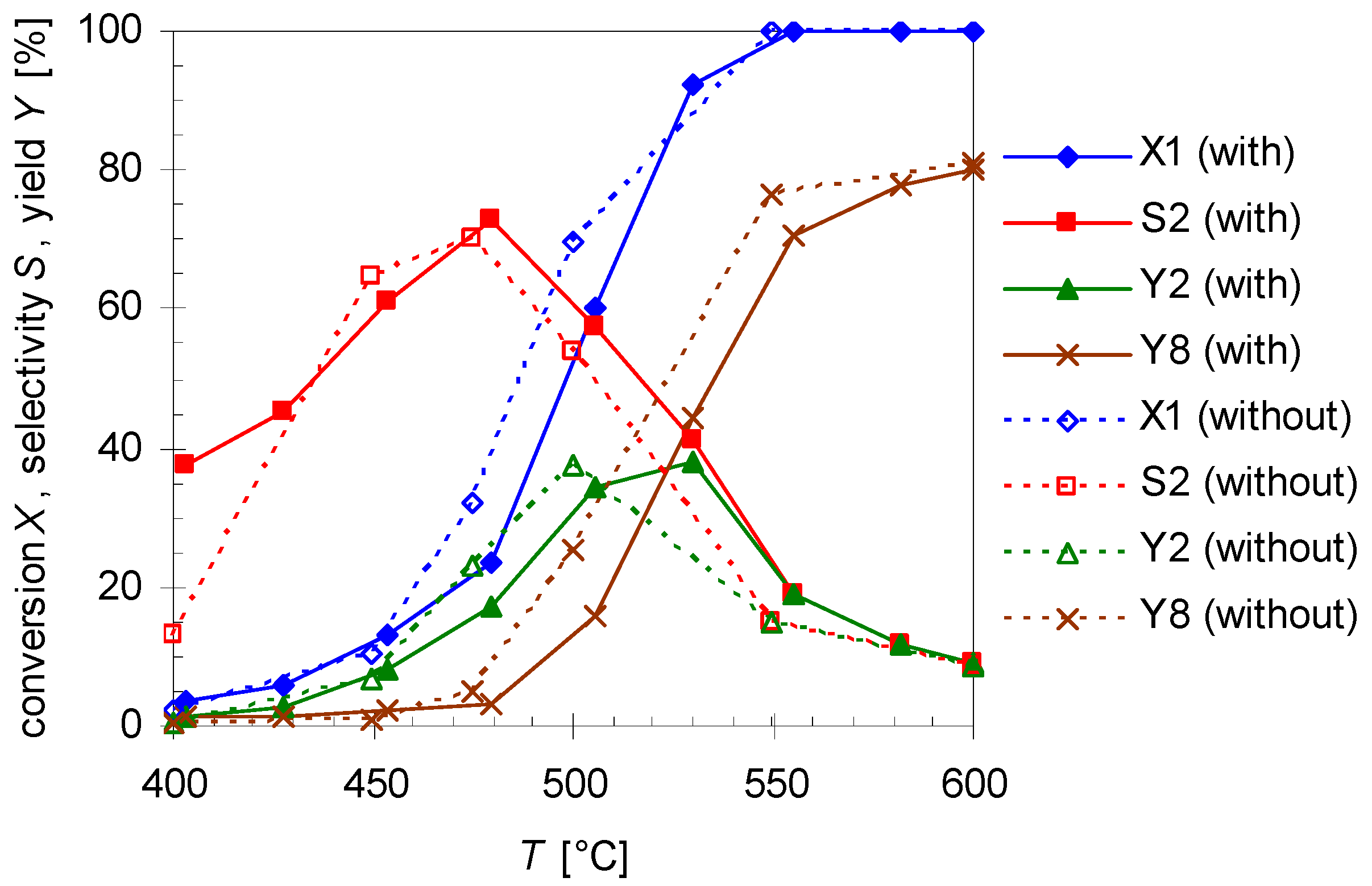
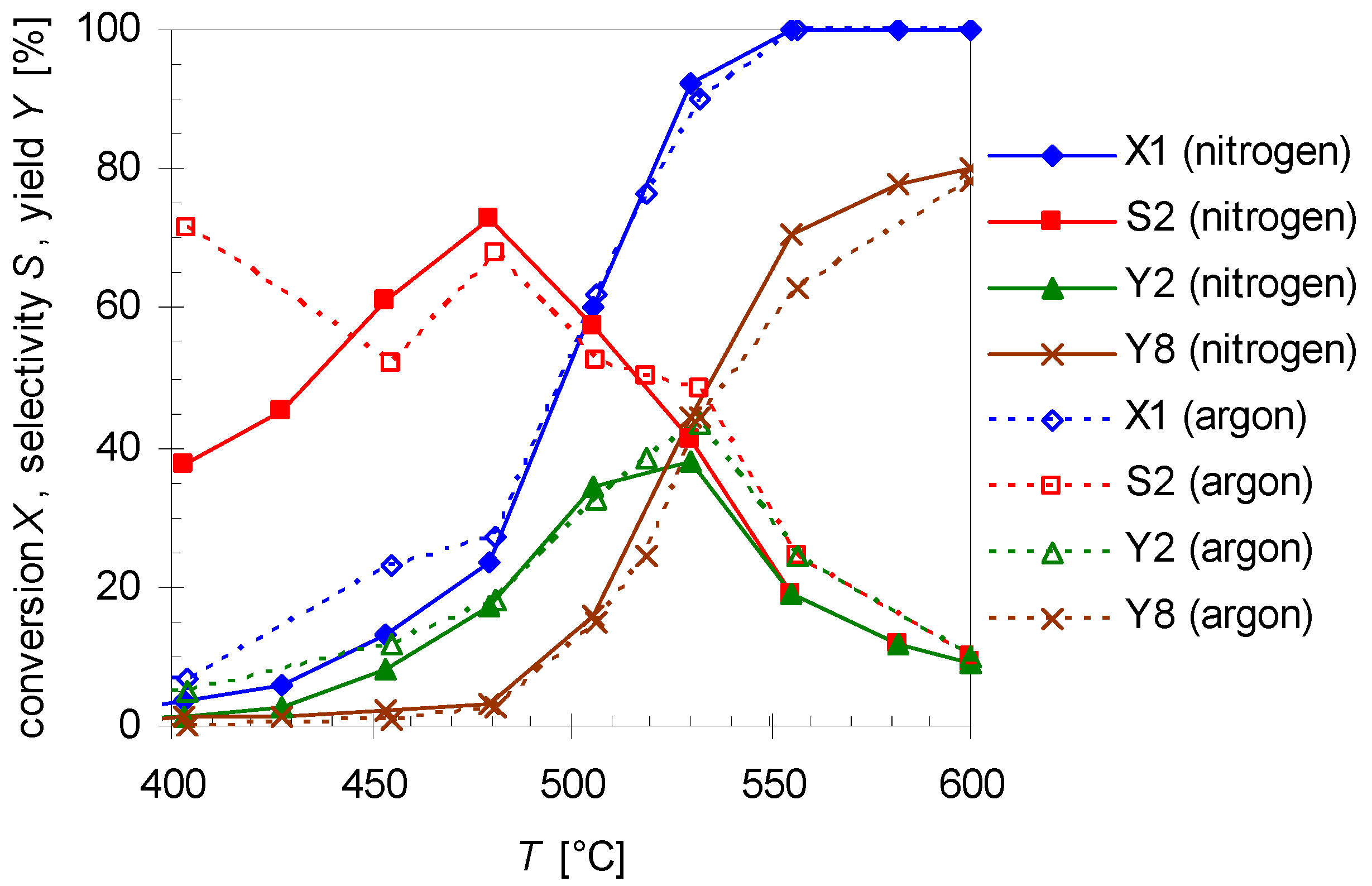
2.5. Thermal Behavior of Pinan-2-ol in the Presence of Additives
3. Experimental
3.1. Chemicals
3.2. Pyrolysis Apparatus
3.3. Pyrolysis Experiments
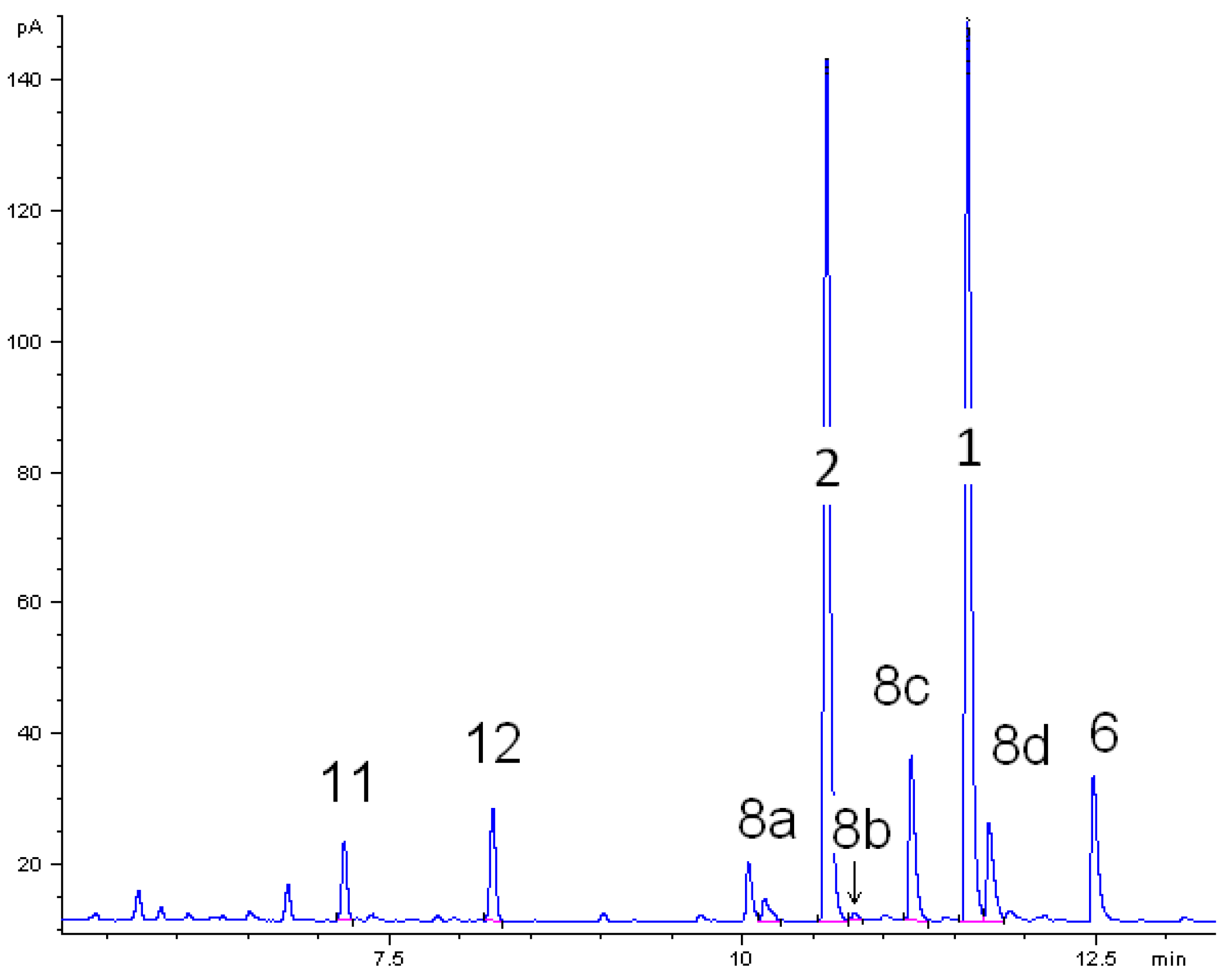
3.4. Analysis of the Pyrolysis Mixtures
4. Conclusions
Conflicts of Interest
References
- Swift, K.A.D. Catalytic transformations of the major terpene feedstocks. Top. Catal. 2004, 27, 143–155. [Google Scholar] [CrossRef]
- Corma, A.; Iborraa, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, W.; Rohdich, F.; Bacher, A. Deoxyxylulose phosphate pathway to terpenoids. Trends Plant Sci. 2001, 6, 78–84. [Google Scholar] [CrossRef]
- Monteiro, J.L.F.; Veloso, C.O. Catalytic conversions of terpenes into fine chemicals. Top. Catal. 2004, 27, 169–180. [Google Scholar] [CrossRef]
- Oldfield, E.; Lin, F.-Y. Terpene biosynthesis: Modularity rules (124 (5), 1150-1163). Angew. Chem. Int. Ed. 2012, 51, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Bonrath, W.; Netscher, T. Catalytic processes in vitamins synthesis and production. Appl. Catal. A 2005, 280, 56–73. [Google Scholar] [CrossRef]
- Breitmaier, E. Terpene; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Batista, P.A.; dePaulaWerner, M.F.; Oliveira, E.C.; Burgos, L.; Pereira, P.; daSilvaBrum, L.F.; Story, G.M.; Santos, A.R.S. The antinociceptive effect of (-)-linalool in models of chronic inflammatory and neuropathic hypersensitivity in mice. J. Pain 2010, 11, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Surburg, H.; Panten, J. Common Fragrance and Flavor Materials; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Suga, T.; Shishibori, T.; Bukeo, M. The biosynthesis of linalool in cinnamomum camphora. Sieb var. Linalooloferum fujita. Bull. Chem. Soc. Jpn. 1972, 45, 1480–1482. [Google Scholar] [CrossRef]
- Tange, K. The biosynthesis of monoterpenoids in higher plants. The biosynthetic pathway leading to the monoterpenoids from amino acids with a carbon-skeleton similar to mevalonic acid. Bull. Chem. Soc. Jpn. 1981, 54, 2763–2769. [Google Scholar] [CrossRef]
- Ohwa, M.; Kogure, T.; Eliel, E.L. An Asymmetric Synthesis of Enantiomerically Pure (S)-(+)-Linalool (3,7-Dimethyl-1,6-octadien-3-ol) and a Formal Synthesis of (R)-(-)-Linalool. J. Org. Chem. 1986, 51, 2599–2601. [Google Scholar] [CrossRef]
- Erman, M.B.; Kane, B.J. Chemistry around pinene and pinane: A facile synthesis of cyclobutanes and oxatricyclo-derivative of pinane from cis- and trans-pinanols. Chem. Biodivers. 2008, 5, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Semikolenov, V.A.; Ilyna, I.I.; Simakova, I.L. Linalool synthesis from α-pinene: Kinetic peculiarities of catalytic steps. Appl. Catal. A 2001, 211, 91–107. [Google Scholar] [CrossRef]
- Sercheli, R.; Ferreira, A.L.B.; Baptistella, L.H.B.; Schuchardt, U. Transition-metal catalyzed autoxidation of cis- and trans-pinane to a mixture of diastereoisomeric pinanols. J. Agric. Food Chem. 1997, 45, 1361–1364. [Google Scholar] [CrossRef]
- Fisher, G.S.; Stinson, J.S.; Goldblatt, L.A. Peroxides from turpentine. Ii. Pinane hydroperoxide. J. Am. Chem. Soc. 1953, 75, 3675–3678. [Google Scholar] [CrossRef]
- Ohloff, G.; Klein, E. Die absolute Konfiguration des Linalools durch Verknüpfung mit dem Pinansystem. Tetrahedron 1962, 18, 37–42. [Google Scholar] [CrossRef]
- Strickler, H.; Ohloff, G.; Kovats, E. Die thermische Cyclisation des (-)-(R)-Linalools. Die Struktur der Plinole und einiger Derivate mit Iridan-Gerüst. Helv. Chim. Acta 1967, 50, 759–797. [Google Scholar] [CrossRef]
- Stolle, A.; Ondruschka, B.; Hopf, H. Thermal rearrangements of monoterpenes and monoterpenoids. Helv. Chim. Acta 2009, 92, 1673–1719. [Google Scholar] [CrossRef]
- Coxon, J.M.; Hartshorn, M.P.; Garland, R.P. The pyrolysis of pinanes. Aust. J. Chem. 1972, 25, 353–360. [Google Scholar] [CrossRef]
- Vandewiele, N.; VanGeem, K.; Reyniers, M.-F.; Marin, G. Kinetic study of the thermal rearrangement of cis- and trans-2-pinanol. J. Anal. Appl. Pyrolysis 2011, 90, 187–196. [Google Scholar] [CrossRef]
- Ilina, I.I.; Simakova, I.L.; Semikolenov, V.A. Kinetics of 2-pinanol isomerization to linalool on the monolith carbon-containing catalyst. Kinet. Catal. 2001, 42, 754–761. [Google Scholar]
- Buddoo, S.; Siyakatshana, N.; Zeelie, B.; Dudas, J. Study of the pyrolysis of 2-pinanol in tubular and microreactor systems with reaction kinetics and modelling. Chem. Eng. Process. 2009, 48, 1419–1426. [Google Scholar] [CrossRef]
- Schmöger, C.; Stolle, A.; Bonrath, W.; Ondruschka, B.; Keller, T.; Jandt, K.D. A practical approach for ambient-pressure hydrogenations using Pd on porous glass. ChemSusChem 2009, 2, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, J.J.; Kuchuk, I.; Hawkins, C.M.; Stine, R. The kinetics, stereochemistry, and deuterium isotope effects in the α-pinene pyrolysis. Evidence for incursion of multiple conformations of a diradical. Tetrahedron 2002, 58, 6943–6950. [Google Scholar] [CrossRef]
- Stolle, A.; Ondruschka, B.; Findeisen, M. Mechanistic and kinetic insights into the thermally induced rearamngement of α-pinene. J. Org. Chem. 2008, 73, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Stolle, A.; Ondruschka, B.; Bonrath, W.; Netscher, T.; Findeisen, M.; Hoffmann, M.M. Thermal isomerization of (+)-cis- and (-)-trans-pinane leading to (-)-β-citronellene and (+)-isocitronellene. Chem.-Eur. J. 2008, 14, 6805–6814. [Google Scholar] [CrossRef] [PubMed]
- Stolle, A.; Bonrath, W.; Ondruschka, B.; Kinzel, D.; González, L. Kinetic model for the thermal isomerization of cis- and trans-pinane. J. Phys. Chem. A 2008, 112, 5882–5892. [Google Scholar] [CrossRef] [PubMed]
- Lemberg, S. Pyrolysis of myrtanol. GB Patent 953776, 1963. [Google Scholar]
- Stolle, A.; Bonrath, W.; Ondruschka, B. Kinetic and mechanistic aspects of myrcene production via thermal-induced β-pinene rearrangement. J. Anal. Appl. Pyrolysis 2008, 83, 26–36. [Google Scholar] [CrossRef]
- Burwell, R.L. The mechanism of the pyrolysis of pinenes. J. Am. Chem. Soc. 1951, 73, 4461–4462. [Google Scholar] [CrossRef]
- Stolle, A.; Ondruschka, B.; Bonrath, W. Comprehensive kinetic and mechanistic considerations for the gas-phase bahavior of pinane-type compounds. Eur. J. Org. Chem. 2007, 2310–2317. [Google Scholar] [CrossRef]
- Stolle, A.; Ondruschka, B. An effort to generalize the thermal isomerization of 6,6-dimethylbicyclo[3.1.1]heptanes and 6,6-dimethylbicyclo[3.1.1]heptenes: Comparative pyrolysis of pinane, α-pinene, and β-pinene. J. Anal. Appl. Pyrolysis 2009, 85, 252–259. [Google Scholar] [CrossRef]
- Banthorpe, D.V.; Whittaker, D. Rearrangements of pinane derivatives. Quart. Rev. 1966, 20, 373–387. [Google Scholar] [CrossRef]
- Woodward, R.B.; Hoffmann, R. Stereochemistry of electrocyclic reactions. J. Am. Chem. Soc. 1965, 87, 825–831. [Google Scholar] [CrossRef]
- Hoffmann, R.; Woodward, R.B. Orbital symmetry control of chemical reactions. Science 1970, 167, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Kinzel, D.; Stolle, A.; Ondruschka, B.; González, L. Quantum chemical investigation of the thermal rearrangement of cis- and trans-pinane. Phys. Chem. Chem. Phys. 2010, 12, 9884–9892. [Google Scholar] [CrossRef] [PubMed]
- Rienäcker, R. Thermische Isomerisierung cyclischer Kohlenwasserstoffe (Chem. Abstracts 1964, 461775). Brennst. Chem. 1964, 45, 206–209. [Google Scholar]
- Lemée, L.; Ratier, M.; Duboudin, J.-G.; Delmond, B. Flash vacuum thermolysis of terpenic compounds in the pinane series. Synth. Commun. 1995, 25, 1313–1318. [Google Scholar] [CrossRef]
- Schulte-Elte, K.H.; Gadola, M.; Ohloff, G. Thermische Umlagerungen von (+)-Isopinocampheol und (-)-Isopinocampheol. Helv. Chim. Acta 1971, 54, 1813–1822. [Google Scholar] [CrossRef]
- Coxon, J.M.; Garland, R.P.; Hartshorn, M.P. The pyrolysis of pinanes, vii. The pyrolysis of some hydroxypinane derivatives. Aust. J. Chem. 1972, 25, 947–957. [Google Scholar] [CrossRef]
- Gebauer, J.; Blechert, S. A short enantiospecific synthesis of (-)-nupharamine. Synlett 2005, 2826–2828. [Google Scholar] [CrossRef]
- Coxon, J.M.; Garland, R.P.; Hartshorn, M.P. The pyrolysis of pinanes, viii. The pyrolysis of some pinanones. Aust. J. Chem. 1972, 25, 2409–2415. [Google Scholar] [CrossRef]
- EuanCant, P.A.; Coxon, J.M.; Hartshorn, M.P. The pyrolysis of pinanes. IX on the mechanism of [σ2+σ2] cycloreversion in pinane thermolysis. Aust. J. Chem. 1975, 18, 391–397. [Google Scholar]
- Weigand, E.F.; Schneider, H.-J. 13C-NMR-spektroskopische und stereochemische Untersuchungen. Org. Mag. Resonance 1979, 12, 637–644. [Google Scholar] [CrossRef]
- Oppolzer, W.; Snieckus, V. Intramolecular ene reactions in organic synthesis. [CrossRef]
- Mayer, C.F.; Crandall, J.K. The pyrolysis of nopinone. J. Org. Chem. 1970, 35, 2688–2690. [Google Scholar] [CrossRef]
- Roy, S.; Chakrabarty, K.; Das, G.K. Comparative study on the transition structures of (3,4) and (3,5) ene cyclizations: A theoretical approach. J. Mol. Struct. 2007, 820, 112–117. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–4 are available from the authors or from commercial sources.


| Reactor geometry a | Without insert cylinder | With hollow insert cylinder b |
|---|---|---|
| dreactor [mm] | 15.2 | 15.2 |
| dinsert [mm] | - | 9.1 |
| Vreactor [cm3] | 36.1 | 23.0 |
| Sreactor [cm2] | 95.2 | 152.4 |
| Scross-section [cm2] | 1.80 | 1.15 |
| S/V-ratio [cm−1] | 2.64 | 6.61 |
| Additive | n1:nsolvent:nadditive | X1 [%] | S2 [%] | Y2 [%] | Y8 [%] | Sp2 [%] |
|---|---|---|---|---|---|---|
| None | 13 | 61 | 8 | 2 | 77 | |
| Dimethylamine | 1:1:0.1 | 8 | 80 | 7 | 1 | 100 |
| Pyridine | 1:1:0.1 | 12 | 57 | 7 | 0 | 58 |
| Water | 1:1:0.1 | 9 | 79 | 7 | 1 | 89 |
| Dimethylamine | 1:1:0.5 | 8 | 78 | 7 | 0 | 78 |
| Pyridine | 1:1:0.5 | 9 | 74 | 7 | 1 | 89 |
| Water | 1:1:0.5 | 9 | 79 | 7 | 1 | 89 |
| Aniline | 1:1:0.5 | 12 | 56 | 6 | 0 | 50 |
| Toluene | 1:1:0.5 | 10 | 67 | 7 | 0 | 70 |
| Additive | n1:nsolvent:nadditive | X1 [%] | S2 [%] | Y2 [%] | Y8 [%] | Sp2 [%] |
|---|---|---|---|---|---|---|
| None | 92 | 41 | 38 | 44 | 89 | |
| Dimethylamine | 1:1:0.1 | 90 | 47 | 42 | 38 | 89 |
| Pyridine | 1:1:0.1 | 89 | 46 | 41 | 35 | 85 |
| Water | 1:1:0.1 | 91 | 47 | 42 | 39 | 89 |
| Dimethylamine | 1:1:0.5 | 88 | 46 | 40 | 34 | 84 |
| Pyridine | 1:1:0.5 | 91 | 44 | 40 | 41 | 89 |
| Water | 1:1:0.5 | 91 | 45 | 41 | 41 | 90 |
| Aniline | 1:1:0.5 | 90 | 45 | 40 | 39 | 88 |
| Toluene | 1:1:0.5 | 90 | 45 | 41 | 39 | 89 |
| Additive | n1:nsolvent:nadditive | T [°C] |
|---|---|---|
| without additive | 1:1:0 | 350–600 |
| Dimethylamine | 1:1:0.1, 1:1:0.5 | 350–600 |
| Pyridine | 1:1:0.1, 1:1:0.5 | 350–600 |
| Water | 1:1:0.1, 1:1:0.5 | 350–600 |
| Aniline | 1:1:0.5 | 425–525 |
| Toluene | 1:1:0.5 | 425–525 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Leiner, J.; Stolle, A.; Ondruschka, B.; Netscher, T.; Bonrath, W. Thermal Behavior of Pinan-2-ol and Linalool. Molecules 2013, 18, 8358-8375. https://doi.org/10.3390/molecules18078358
Leiner J, Stolle A, Ondruschka B, Netscher T, Bonrath W. Thermal Behavior of Pinan-2-ol and Linalool. Molecules. 2013; 18(7):8358-8375. https://doi.org/10.3390/molecules18078358
Chicago/Turabian StyleLeiner, Janne, Achim Stolle, Bernd Ondruschka, Thomas Netscher, and Werner Bonrath. 2013. "Thermal Behavior of Pinan-2-ol and Linalool" Molecules 18, no. 7: 8358-8375. https://doi.org/10.3390/molecules18078358
APA StyleLeiner, J., Stolle, A., Ondruschka, B., Netscher, T., & Bonrath, W. (2013). Thermal Behavior of Pinan-2-ol and Linalool. Molecules, 18(7), 8358-8375. https://doi.org/10.3390/molecules18078358