Separation and Identification of 1,2,4-Trihydroxynaphthalene-1-O-glucoside in Impatiens glandulifera Royle
Abstract
:1. Introduction
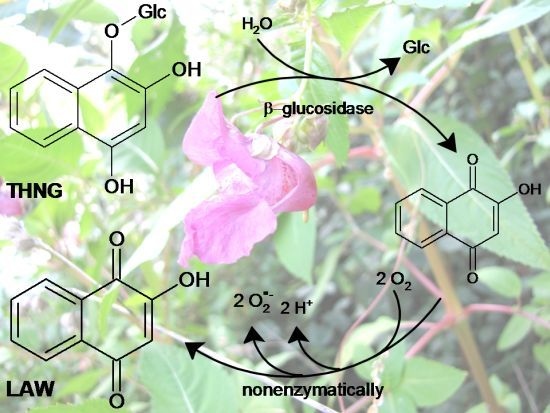
2. Results and Discussion





| Year of collection | Part of plant | Drying | Storage | Solvents | THNG | A | B |
|---|---|---|---|---|---|---|---|
| 2008 | root | RT | RT | MeOH | ND | 48 | 8 |
| 2008 | root | −18 °C+LY | RT | MeOH | 204 | 92 | 13 |
| 2008 | root | −18 °C+LY | 4 °C | MeOH | 210 | 115 | 7 |
| 2011 | stem | 40 °C | RT | MeOH | 2 | 53 | 19 |
| 2011 | stem | −18 °C+LY | RT | MeOH | 148 | 74 | 11 |
| 2011 | stem | −18 °C+LY | 4 °C | MeOH | 176 | 70 | 10 |
| 2011 | stem | −18 °C+LY | −18 °C | MeOH | 200 | 118 | 15 |
| 2011 | root | −18 °C+LY | −18 °C | MeOH | 826 | 143 | 51 |
| 2011 | leaf | −18 °C+LY | −18 °C | MeOH | 247 | ND | 69 |
| 2013 | leaf | N2(l)+LY | WS | MeOH | 230 | ND | 111 |
| 2013 | stem | N2(l)+LY | WS | MeOH | 202 | 148 | 20 |
| 2013 | root | N2(l)+LY | WS | MeOH | 1359 | 115 | 29 |
| 2013 | leaf | N2(l)+LY | WS | H2O | ND | 499 | 1301 |
| 2013 | stem | N2(l)+LY | WS | H2O | ND | 356 | 58 |
| 2013 | root | N2(l)+LY | WS | H2O | 98 | 2277 | 81 |
| THNG | A | B | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Year of collection | Part of plant | Drying | Storage | H2O | MeOH | H2O | MeOH | H2O | MeOH |
| 2008 | root | RT | RT | ND | ND | 46 | 16 | 4 | 1 |
| 2008 | root | LY | RT | ND | ND | 740 | 335 | 17 | 23 |
| 2008 | root | LY | 4 °C | ND | ND | 1121 | 484 | 15 | 20 |
| 2011 | stem | 40 °C | RT | ND | ND | 128 | 47 | ND | ND |
| 2011 | stem | LY | RT | ND | ND | 321 | 235 | 43 | 25 |
| 2011 | stem | LY | 4 °C | ND | ND | 263 | 177 | 48 | 24 |
| 2011 | stem | LY | −18 °C | ND | ND | 319 | 191 | 54 | 24 |


3. Experimental
3.1. Standards
3.2. Sample Preparation
- 1)
- Extraction with 100% methanol for 30 min at the room temperature under continuous shaking, after centrifugation the sediment was washed further twice with methanol and the supernatants were combined.
- 2)
- Extraction with distilled water under the same conditions as with methanol and after centrifugation the sediment was washed twice with water, supernatants were combined, then the sediment was extracted with methanol for 30 min at the room temperature under continuous shaking, the sediment after centrifugation was washed twice with methanol and methanolic extracts were combined (Table 2).
- 3)
- For NMR measurements the lyophilized roots were not extracted with methanol, but with ethyl acetate. Ethyl acetate fraction was then evaporated in a vacuum evaporator to dryness and the sample was dissolved in small volume of methanol. Extraction with ethyl acetate is not as effective as extraction with methanol, but the resulting extract is relatively clean, containing only a minor impurities. During the extraction with ethyl acetate very polar compounds and free sugars do not pass into extract. After evaporation of the ethyl acetate and dissolution of the residue in a small amount of methanol (the mixture must be filtrated or centrifuged) we will obtain the extract which is almost free from non polar compounds, e.g., lipids, sterols, etc. Before NMR measurements the methanol was evaporated and the sample was dissolved in deuterochloroform.
3.3. Samples Analysis
3.3.1. HPLC Analysis
3.3.2. LC-MS Analysis
3.3.3. NMR measurement
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lobstein, A.; Brenne, X.; Feist, E.; Metz, N.; Weniger, B.; Anton, R. Quantitative determination of naphthoquinones of Impatiens species. Phytochem. Anal. 2001, 12, 202–205. [Google Scholar] [CrossRef]
- Šerá, B.; Vrchotová, N.; Tříska, J. Phenolic compounds in the leaves of alien and native Impatiens plants. In Proceedings of the Introduction and Spread of Invasive Species, Berlin, Germany, 9–11 June 2005.
- Ashnagar, A.; Shiri, A. Isolation and characterization of 2-hydroxy-1,4-naphthoquinone (lawsone) from the powdered leaves of henna plant marketed in Ahwaz city of Iran. Int. J. ChemTech. Res. 2011, 3, 1941–1944. [Google Scholar]
- Vrchotová, N.; Tříska, J.; Jílek, R. Separation of quinones from the roots of Impatients glandulifera on the SPE columns. In Proceedings of the Italian meeting on lignocellulosic chemistry 6- Analytical Techniques for biorefineries, Viterbo, Italy, September 2011.
- Takeda, Y.; Fatope, M.O. New phenolic glucosides from Lawsonia inermis. J. Nat. Prod. 1988, 51, 725–729. [Google Scholar] [CrossRef]
- Hsouna, A.B.; Trigui, M.; Culili, G.; Blafne, Y.; Jaoua, S. Antioxidant constituents from Lawsonia inermis leaves: Isolation, structure elucidation and antioxidative kapacity. Food Chem. 2011, 125, 193–200. [Google Scholar] [CrossRef]
- Budzianowski, J. Naphthoquinones of Drosera spathulate from in vitro culture. Phytochemistry 1995, 40, 1145–1148. [Google Scholar] [CrossRef]
- Hedin, P.A.; Collum, D.H.; Langhans, V.E.; Graves, C.H. Distribution of muflone and related compounds in pecan and their effect on Fusicladium effusum. J. Agric. Food Chem. 1980, 28, 340–342. [Google Scholar] [CrossRef]
- Son, J.K. Isolation and structure determination of a new tetralone glucoside from the roots of Juglans mandshurica. Arch. Pharm. Res. 1995, 18, 203–205. [Google Scholar] [CrossRef]
- Steinerová, N.; Cudlín, J.; Vaněk, Z. Glucosidation of 2-hydroxy- and 5-hydroxy-1,4-naphthoquinone. Collect. Czech. Chem. Commun. 1980, 45, 2684–2687. [Google Scholar]
- Babula, P.; Adam, V.; Kizek, R.; Sladký, Z.; Havel, L. Naphthoquinones as allelochemical triggers of programmed cell death. Environ. Exp. Bot. 2009, 65, 330–337. [Google Scholar] [CrossRef]
- Vrchotová, N.; Šerá, B.; Krejčová, J. Allelopathic activity of extracts from Impatients species. Plant Soil Environ. 2011, 57, 57–60. [Google Scholar]
- Budzianowski, J. Naphthoquinone glucosides of Drosera gigantea from in vitro cultures. Planta Med. 2000, 66, 667–669. [Google Scholar]
- Ding, Z.S.; Jiang, F.S.; Chen, N.P.; Lv, G.Y.; Zhu, Ch.G. Isolation and identification of an anti-tumor component from leaves of Impatiens balsamina. Molecules 2008, 13, 220–229. [Google Scholar] [CrossRef]
- Müller, U.; Lingens, F. Degradation of 1,4-naphthoquinones by Pseudomonas putida. Biol. Chem. Hoppe Seyler. 1988, 369, 1031–1043. [Google Scholar] [CrossRef]
- Panjchayupakaranant, P.; Noguchi, H.; De-Eknamkul, W.; Sankawa, U. Naphthoquinones and coumarins from Impatiens balsamina root cultures. Phytochemistry 1995, 40, 1141–1143. [Google Scholar] [CrossRef]
- International Union of Pure and Applied Chemistry. Guidelines for data acquisition and data duality evaluation in environmental chemistry. Anal. Chem. 1980, 52, 2242–2249. [CrossRef]
- Sample Availability: Samples of the extracts are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tříska, J.; Vrchotová, N.; Sýkora, J.; Moos, M. Separation and Identification of 1,2,4-Trihydroxynaphthalene-1-O-glucoside in Impatiens glandulifera Royle. Molecules 2013, 18, 8429-8439. https://doi.org/10.3390/molecules18078429
Tříska J, Vrchotová N, Sýkora J, Moos M. Separation and Identification of 1,2,4-Trihydroxynaphthalene-1-O-glucoside in Impatiens glandulifera Royle. Molecules. 2013; 18(7):8429-8439. https://doi.org/10.3390/molecules18078429
Chicago/Turabian StyleTříska, Jan, Naděžda Vrchotová, Jan Sýkora, and Martin Moos. 2013. "Separation and Identification of 1,2,4-Trihydroxynaphthalene-1-O-glucoside in Impatiens glandulifera Royle" Molecules 18, no. 7: 8429-8439. https://doi.org/10.3390/molecules18078429
APA StyleTříska, J., Vrchotová, N., Sýkora, J., & Moos, M. (2013). Separation and Identification of 1,2,4-Trihydroxynaphthalene-1-O-glucoside in Impatiens glandulifera Royle. Molecules, 18(7), 8429-8439. https://doi.org/10.3390/molecules18078429