Oligo-Carrageenans Enhance Growth and Contents of Cellulose, Essential Oils and Polyphenolic Compounds in Eucalyptus globulus Trees
Abstract
:1. Introduction
2. Results and Discussion
2.1. Oligo-Carrageenans Increased Growth, Net Photosynthesis and Contents of Chlorophylls in Eucalyptus Trees

2.2. Oligo-Carrageenans Increased Holo-Cellulose and α-Cellulose Contents in Eucalyptus Trees

2.3. Oligo-Carrageenans Increased Content of Essential Oils in Eucalyptus Trees

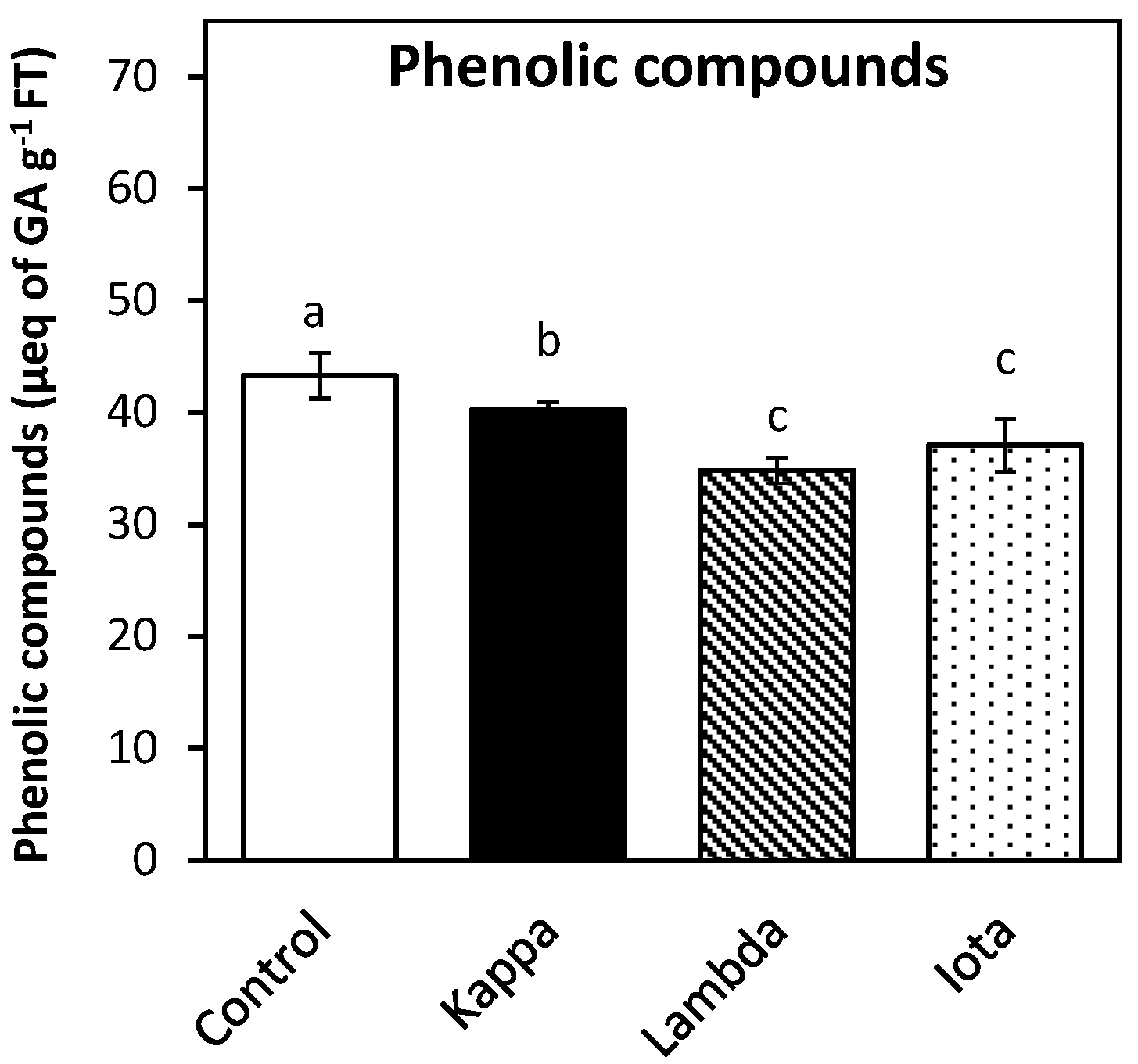
2.4. Oligo-Carrageenans Changed the Level of Polyphenolic Compounds in Eucalyptus Trees
| PPC contents (mg g−1 of fresh tissue) | ||||
|---|---|---|---|---|
| Control | Kappa | Lambda | Iota | |
| Ellagic acid | 0.6 | 0.6 | 0.9 | 0.6 |
| Quercetin | 2.5 | 1.6 | 1.5 | 2.5 |
| Morin | 1.7 | 0.1 | 2.0 | 1.4 |
| Rutin | 0.1 | 0.3 | 0.0 | 0.5 |
| Luteolin | 2.6 | 1.7 | 1.9 | 2.4 |
| Genistein | 0.2 | 0.8 | 1.3 | 1.4 |
| Sinapic acid | 1.7 | 1.2 | 1.4 | 1.7 |
3. Experimental
3.1. Preparation of Oligo-Carrageenans
3.2. Plant Culture, Treatment and Measurement of Height and Trunk Diameter
3.3. Determination of Net Photosynthesis
3.4. Determination of Chlorophyll a and b Contents
3.5. Determination of Holo-Cellulose and α-Cellulose Contents
3.6. Determination of Essential Oils Content
3.7. Determination of Total Phenolic Compounds
3.8. Analysis of Methanol-Soluble Polyphenolic Compounds
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Australian Native Plant Society. Available online: http://www.anpsa.org.au (accessed on 20 May 2013).
- Somerville, C. Cellulose synthesis in higher plants. Annu. Rev. Cell Dev. Biol. 2006, 22, 53–68. [Google Scholar] [CrossRef]
- Delmer, D.P. Cellulose biosynthesis: exciting times for a difficult field of study. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1999, 50, 245–276. [Google Scholar] [CrossRef]
- Doblin, M.A.; Kureck, L.; Jacob-Wilk, D.; Delmer, D.P. Cellulose biosynthesis in plants: From genes to rosettes. Plant. Cell Physiol. 2002, 43, 1407–1420. [Google Scholar] [CrossRef]
- Singh, H.P.; Mittal, S.; Kaur, S.; Batish, D.R.; Kohli, R.K. Characterization and antioxidant activity of essential oils from fresh and decaying leaves of Eucalyptus tereticornis. J. Agric. Food Chem. 2009, 57, 6962–6996. [Google Scholar] [CrossRef]
- Tohidpour, A.; Sattari, M.; Ornidbaigi, R.; Yadegar, A.; Nazerri, J. Antibacterial effect of essential oils from two medicinal plants against methicillin-resistant Staphylococcus aureus (MRSA). Phytomedicine 2010, 17, 142–145. [Google Scholar]
- Ammon, D.G.; Barton, A.F.M.; Clarke, D.A.; Tjandra, J. Rapid and accurate determination of terpenes in the leaves of eucalyptus species. Analyst 1985, 110, 921–924. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Sporer, F.; Reichling, J.; Wink, M. Antibacterial activity of essential oils from eucalyptus and of selected components against multidrug-resistance bacterial pathogens. Pharm. Biol. 2011, 49, 893–899. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef]
- Morcia, C.; Malnati, M.; Terzi, V. In vitro antifungal activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxygenic plant pathogens. Food Addit. Contamin. 2012, 29, 415–422. [Google Scholar]
- Schnitzler, P.; Shön, K.; Reichling, J. Antiviral activity of Australian tree tea oil and eucalyptus oil against herpes simplex virus in cell culture. Pharmazie 2001, 56, 343–347. [Google Scholar]
- Santos, S.A.; Freire, C.S.; Domingues, M.R.; Silvestre, A.J.; Pascoal-Neto, C. Characterization of phenolic components in polar extracts of Eucalyptus globulus Labill. Bark by high performance liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2011, 59, 9386–9393. [Google Scholar] [CrossRef]
- Zhou, L.D.; Jiang, W.; Qin, Z.; Zhao, S.; Qiu, M.; Wu, J. Two ellagic acid glycosides from Gleditsia sinensis Lam. with antifungal activity on Magnaporthe grisea. Nat. Prod. Res. 2007, 21, 303–309. [Google Scholar] [CrossRef]
- Weinderbörner, M.; Hindorf, H.; Tsotsonos, P.; Hegge, H. Antifungal activity of isoflavonoids in different reduce stages on Rhizoctonia solani and Sclerotium rolfsi. Phytochemistry 1990, 29, 801–803. [Google Scholar] [CrossRef]
- Almeida, I.F.; Fernandes, E.; Lima, J.L.; Valentao, P.; Andrade, P.B.; Seabra, R.M.; Costa, P.C.; Bahia, M.F. Oxygen and nitrogen reactive species are effectively scavenged by Eucalyptus globulus leaf water extract. J. Med. Food 2009, 12, 175–183. [Google Scholar] [CrossRef]
- González, A.; Castro, J.; Vera, J.; Moenne, A. Seaweed oligosaccharides stimulate plant growth by enhancing carbon and nitrogen assimilation, basal metabolism and cell division. J. Plant. Growth Regul. 2013, 32, 443–448. [Google Scholar] [CrossRef]
- Vera, J.; Castro, J.; González, A.; Moenne, A. Seaweed polysaccharides and oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef]
- Castro, J.; Vera, J.; González, A.; Moenne, A. Oligo-carrageenans stimulate growth by enhancing photosynthesis, basal metabolism and cell cycle in tobacco plants (var. Burley). J. Plant Growth Regul. 2012, 31, 173–185. [Google Scholar] [CrossRef]
- Moenne, A. Composition and method to stimulate plant growth and defense against pathogens in plants. U.S. Patent US20100173779 A1, 2009. [Google Scholar]
- Vera, J.; Castro, J.; González, A.; Moenne, A. Oligo-carrageenans induced a long-term and broad-range protection against pathogens in tobacco plants (var. Xanthi). Physiol. Mol. Plant. Pathol. 2012, 79, 1–39. [Google Scholar] [CrossRef]
- Kopriva, S.; Suter, M.; von Ballmoos, O.; Hesse, H.; Krähenbühl, U.; Rennenberg, H.; Brunold, C. Interaction of sulfate metabolism with carbon nitrogen metabolism in Lemna minor. Plant. Physiol. 2002, 130, 1406–1413. [Google Scholar] [CrossRef]
- Lillo, C. Signaling cascades integrating light-enhanced nitrate metabolism. Biochem. J. 2008, 415, 11–19. [Google Scholar] [CrossRef]
- Ullanowska, K.; Tkaczyk, A.; Konopa, G.; Wegrzyn, G. Differential antibacterial activity of genistein arising from global inhibition of DNA, RNA and protein synthesis in some bacterial strains. Arch. Microbiol. 2006, 184, 271–278. [Google Scholar] [CrossRef]
- Shetty, R.; Fretté, X.; Jensen, B.; Shetty, N.P.; Jensen, J.D.; Jorgensen, H.J.L.; Newman, M.A.; Christensen, L.P. Silicon-induced changes in antifungal phenolic acids, flavonoids, and key phenylpropanoid genes during the interaction of miniature roses and the biotrophic pathogen Podosphaera pannosa. Plant Physiol. 2011, 157, 2194–2205. [Google Scholar] [CrossRef] [Green Version]
- Arima, H.; Ashida, H.; Danno, G. Rutin-enhanced antibacterial activities of flavonoids against Bacillus cereus and Salmonella enteritidis. Biosc. Biotechnol. Biochem. 2002, 66, 1009–1014. [Google Scholar] [CrossRef]
- Loo, W.T.Y.; Jin, L.J.; Cheung, M.N.B.; Cho, L.W.C. Evaluation of ellagic acid on the activities of oral bacteria with the use of adenosine triphosphate (ATP) bioluminescence assay. Afr. J. Biotechnol. 2010, 9, 3938–3943. [Google Scholar]
- Santos, S.A.; Villaverde, J.J.; Silva, C.M.; Neto, C.P.; Silvestre, A.J. Supercritical fluid extraction of phenolic compounds of Eucaliptus globulus Labill bark. J. Supercrit. Fluids 2012, 71, 71–79. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b in leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar]
- Yokohama, T.; Kadla, J.F.; Chang, H.M. Microanalytical method for the characterization of fiber components and morphology of woody plants. J. Agric. Food Chem. 2002, 50, 1040–1044. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Prentice Hall, Inc.: Englewood Cliffs: NJ, USA, 1999. [Google Scholar]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
González, A.; Contreras, R.A.; Moenne, A. Oligo-Carrageenans Enhance Growth and Contents of Cellulose, Essential Oils and Polyphenolic Compounds in Eucalyptus globulus Trees. Molecules 2013, 18, 8740-8751. https://doi.org/10.3390/molecules18088740
González A, Contreras RA, Moenne A. Oligo-Carrageenans Enhance Growth and Contents of Cellulose, Essential Oils and Polyphenolic Compounds in Eucalyptus globulus Trees. Molecules. 2013; 18(8):8740-8751. https://doi.org/10.3390/molecules18088740
Chicago/Turabian StyleGonzález, Alberto, Rodrigo A. Contreras, and Alejandra Moenne. 2013. "Oligo-Carrageenans Enhance Growth and Contents of Cellulose, Essential Oils and Polyphenolic Compounds in Eucalyptus globulus Trees" Molecules 18, no. 8: 8740-8751. https://doi.org/10.3390/molecules18088740




