Two Anti-inflammatory Steroidal Saponins from Dracaena angustifolia Roxb.
Abstract
:1. Introduction
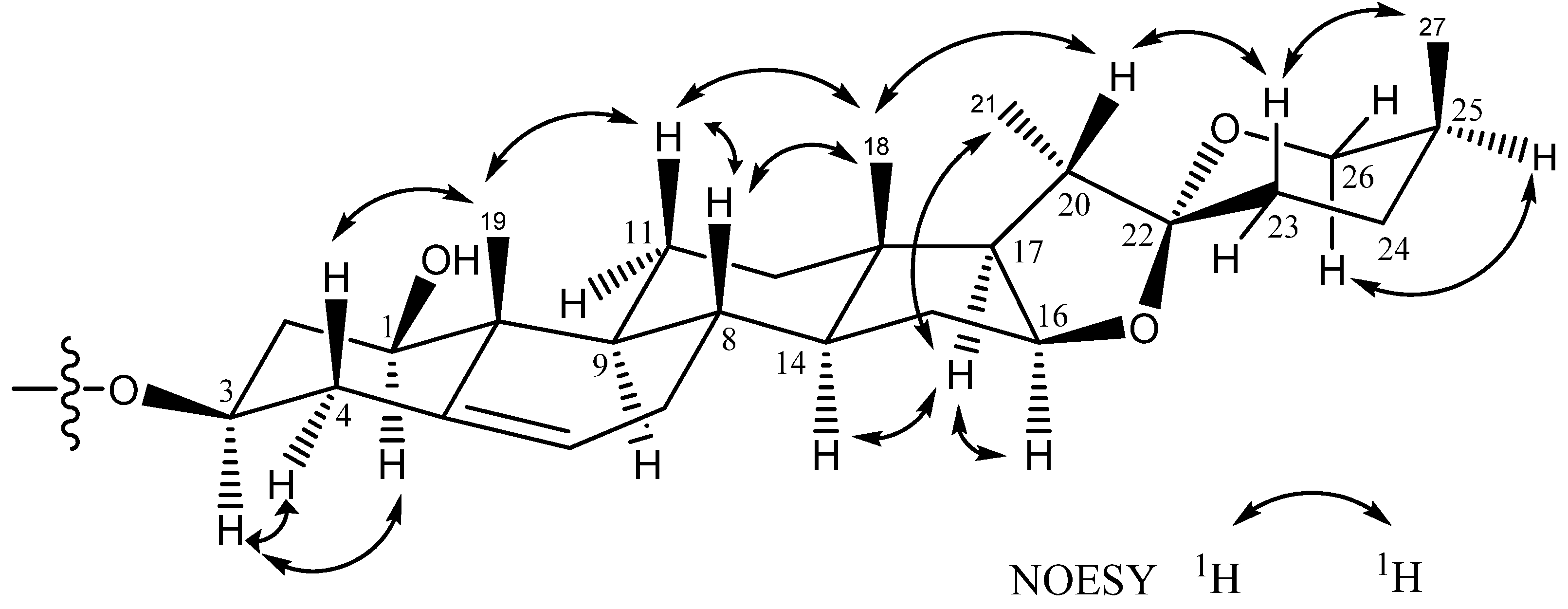
2. Results and Discussion
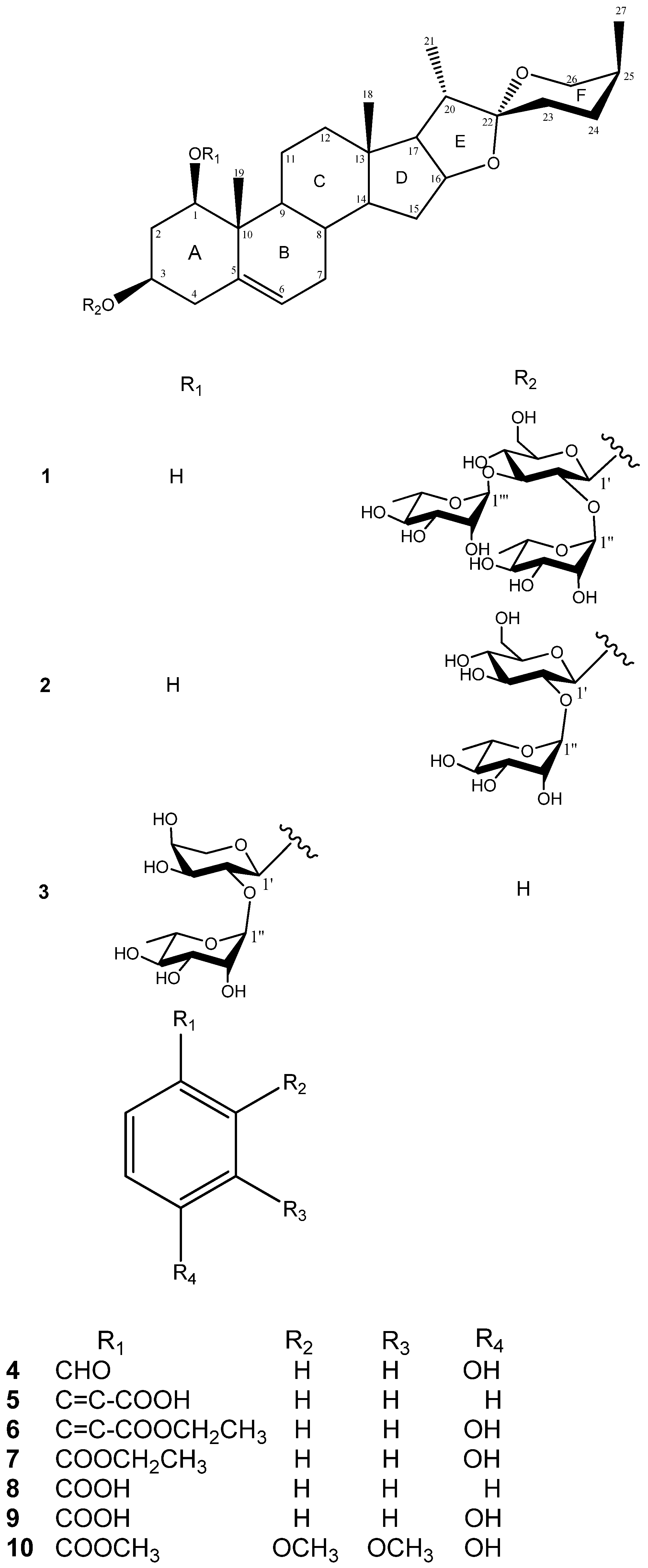
| 1 δH | δC | 2 δH | δC | 3 δH | δC | |||
|---|---|---|---|---|---|---|---|---|
| 1 | 3.68 (dd, 11.6, 3.9) | 78.1 | 3.70 (dd, 12.4, 4.2) | 78.2 | 3.86 (dd, 12.1, 3.7) | 83.9 | ||
| 2 | 2.58 (br d, 12.5), | 41.9 | 2.63 (br d, 12.4), | 41.1 | 2.72 (br d, 12.4), | 37.7 | ||
| 2.28 (q, 11.8) | 2.36 (q, 11.9) | 2.36 (q, 11.8) | ||||||
| 3 | 4.02 (m) | 75.0 | 4.06 (m) | 75.1 | 3.86 (m) | 68.5 | ||
| 4 | 2.77 (m), 2.75 (m) | 39.4 | 2.84 (m), 2.82 (m) | 39.7 | 2.61, 2.57 | 44.1 | ||
| 5 | - | 139.3 | - | 139.4 | - | 139.9 | ||
| 6 | 5.53 (d, 5.1) | 125.5 | 5.54 (d, 6.2) | 125.4 | 5.57 (d, 5.2) | 125.0 | ||
| 7 | 1.90 (m), 1.54 (br d, 9.6) | 32.7 | 1.92 (m), 1.57 (m) | 32.6 | 1.89 (m), 1.55 (m) | 32.3 | ||
| 8 | 1.58 (m) | 33.2 | 1.59 (m) | 33.2 | 1.57 (m) | 33.4 | ||
| 9 | 1.32 (m) | 51.4 | 1.36 (m) | 51.5 | 1.50 (m) | 50.7 | ||
| 10 | - | 44.0 | - | 44.1 | - | 43.2 | ||
| 11 | 2.83 (dd, 11.3, 3.5), 1.70 (m) | 24.4 | 2.85 (m) | 24.4 | 2.91 (m) | 24.3 | ||
| 1.75 (m) | 1.63 (m) | |||||||
| 12 | 1.72 (m), 1.21 (m) | 40.7 | 1.73 (m), 1.23 (m) | 40.8 | 1.54 (m), 1.28 (m) | 40.6 | ||
| 13 | - | 40.4 | - | 40.5 | - | 40.5 | ||
| 14 | 1.13 (m) | 57.1 | 1.13 (m) | 57.1 | 1.13 (m) | 57.1 | ||
| 15 | 2.05 (m), 1.47 (m) | 32.5 | 2.07 (m), 1.49 (m) | 32.7 | 2.01 (m), 1.39 (m) | 32.7 | ||
| 16 | 4.51 (dd, 14.5, 7.9) | 81.6 | 4.51 (dd, 14.7, 8.0) | 81.4 | 4.50 (dd, 14.5, 7.5) | 81.5 | ||
| 17 | 1.77 (t, 7.9) | 63.2 | 1.78 (t, 8.0) | 63.3 | 1.71 (t, 7.5) | 63.1 | ||
| 18 | 0.87 (s) | 16.8 | 0.90 (s) | 16.8 | 0.84 (s) | 17.0 | ||
| 19 | 1.34 (s) | 14.0 | 1.37 (s) | 14.0 | 1.44 (s) | 15.3 | ||
| 20 | 1.87 (m) | 42.7 | 1.88 (m) | 42.8 | 1.84 (m) | 42.7 | ||
| 21 | 1.08 (d, 7.0) | 15.1 | 1.11 (d, 6.9) | 15.1 | 1.10 (d, 7.0) | 15.1 | ||
| 22 | - | 110.0 | - | 110.0 | - | 110.0 | ||
| 23 | 1.88 (m), 1.43 (m) | 26.7 | 1.91 (m), 1.45 (m) | 26.7 | 1.86 (m), 1.40 (m) | 26.7 | ||
| 24 | 2.11 (m), 1.36 (m) | 26.5 | 2.15 (m), 1.37 (m) | 26.5 | 2.11 (m), 1.34 (m) | 26.5 | ||
| 25 | 1.58 (m) | 27.8 | 1.61 (m) | 27.8 | 1.55 (m) | 27.8 | ||
| 26 | 4.05 (br d, 11.5), | 65.3 | 4.09 (br d, 10.8), | 65.3 | 4.06 (br d, 10.8), | 65.3 | ||
| 3.34 (d, 11.5) | 3.36 (d, 10.8) | 3.35 (d, 10.8) | ||||||
| 27 | 1.06 (d, 7.0) | 16.5 | 1.07 (d, 7.0) | 16.6 | 1.06 (d, 7.1) | 16.6 | ||
| 3-O-β-D-Glc | 3-O-β-D-Glc | 3-O-α-L-Ara | ||||||
| 1' | 4.91 (d, 7.7) | 100.2 | 5.06 (d, 7.3) | 100.7 | 4.72 d (7.0) | 100.7 | ||
| 2' | 3.99 (dd, 8.0, 7.7) | 78.3 | 4.23 (m) | 78.0 | 4.59 (m) | 75.4 | ||
| 3' | 4.14 (t, 8.0) | 87.6 | 4.26 (m) | 79.9 | 4.08 (m) | 76.2 | ||
| 4' | 4.03 (m) | 70.2 | 4.16 (t, 8.6) | 72.9 | 4.06 (m) | 70.4 | ||
| 5' | 3.78 (m) | 78.3 | 3.88 (m) | 78.5 | 4.25 (m)3.66 (d, 11.0) | 67.6 | ||
| 6' | 4.38 (br d, 11.5), | 62.4 | 4.46 (dd, 11.8, 1.9), | 62.8 | - | |||
| 4.31 (dd, 11.5, 5.9) | 4.31 (m) | |||||||
| α-L-RhaI | α-L-Rha | α-L-Rha | ||||||
| 1'' | 5.82 (br s) | 104.1 | 6.36 (br s) | 102.3 | 6.31 (br s) | 101.9 | ||
| 2'' | 4.73 (m) | 72.9 | 4.80 (m) | 72.8 | 4.70 (br d, 3.5) | 72.9 | ||
| 3'' | 4.52 (m) | 73.0 | 4.64 (dd, 9.2, 3.1) | 73.1 | 4.63 (dd, 9.3, 3.5) | 72.8 | ||
| 4'' | 4.46 (m) | 74.0 | 4.34 (m) | 74.4 | 4.31 (dd, 9.3, 9.3) | 74.8 | ||
| 5'' | 4.76 (dq, 8.9, 6.2) | 70.1 | 4.99 (m) | 69.7 | 4.85 (dq, 9.3, 6.2) | 69.5 | ||
| 6'' | 1.62 (d, 6.2) | 18.6 | 1.71 (d, 6.2) | 18.9 | 1.73 (d, 6.2) | 19.3 | ||
| α-L-RhaII | ||||||||
| 1''' | 5.73 (br s) | 102.8 | ||||||
| 2''' | 4.81 (br s) | 72.7 | ||||||
| 3''' | 4.46 (m) | 72.8 | ||||||
| 4''' | 4.29 (m) | 73.8 | ||||||
| 5''' | 4.86 (dq, 9.1, 6.2) | 70.9 | ||||||
| 6''' | 1.68 (d, 6.2) | 18.9 |

| Compound | Superoxide anion IC50 (μM) a | Elastase release IC50 (μM) a |
|---|---|---|
| 1 | 26.39 ± 1.63 | 3.94 ± 0.19 |
| 2 | 18.55 ± 0.23 | 1.74 ± 0.25 |
| LY294002 | 2.00 ± 0.59 | 4.94 ± 1.69 |
3. Experimental
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectral Data
3.5. Acid Hydrolysis of 1–2
3.6. Superoxide Generation and Elastase Release by Human Neutrophils
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ying, S.S. Flora of Taiwan, 2nd ed.; Editorial Committee of the Flora of Taiwan: Taipei, Taiwan, 1994. [Google Scholar]
- Chi, V.V. In Vietnamese Medicinal Plants; Medicine publisher: Hanoi, Vietnam, 2001. [Google Scholar]
- Kougan, G.B.; Miyamoto, T.; Tanaka, C.; Paululat, T.; Mirjolet, J.F.; Duchamp, O.; Sondengam, B.L.; Lacaille-Dubois, M.A. Steroidal saponins from two species of Dracaena. J. Nat. Prod. 2010, 73, 1266–1270. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Y.J.; Li, X.C.; Jacob, M.R.; Yang, C.R. Steroidal saponins from fresh stems of Dracaena angustifolia. J. Nat. Prod. 2010, 73, 1524–1528. [Google Scholar] [CrossRef]
- Gupta, D.; Bleakley, B.; Gupta, R.K. Dragon’s blood: Botany, Chemistry and therapeutic uses. J. Ethnopharml. 2008, 115, 361–380. [Google Scholar] [CrossRef]
- Minh, C.V.; Dat, N.T.; Dang, N.H.; Nam, N.H.; Ban, N.K.; Tuyen, N.V.; Huong, M.; Huong, T.T.; Kiem, P.V. Unusual 22S-spirostane steroids from Dracaena cambodiana. Nat. Prod. Commun. 2009, 4, 1197–2000. [Google Scholar]
- Luo, Y.; Wang, H.; Xu, X.; Mei, W.; Dai, H. Antioxidant phenolic compounds of Dracaena cambodiana. Molecules 2010, 6, 8904–8914. [Google Scholar]
- Yi, T.; Chen, H.B.; Zhao, Z.Z.; Yu, Z.L.; Jiang, Z.H. Comparison of the chemical profiles and anti-platelet aggregation effects of two “dragon’s blood” drugs used in traditional Chinese medicine. J. Ethnopharm. 2011, 133, 796–802. [Google Scholar] [CrossRef]
- Liu, J.; Mei, W.L.; Wu, J.; Zhao, Y.X.; Peng, M.; Dai, H.F. A new cytotoxic homoisoflavonoid from Dracaena cambodiana. J. Asian Nat. Prod. Res. 2009, 11, 192–195. [Google Scholar] [CrossRef]
- Vesela, D.; Marek, R.; Ubik, K.; Lunerova, K.; Sklenar, V.; Suchy, V. Dracophane, a metacyclophane derivative from the resin of Dracaena cinnabari Balf. Phytochemistry 2002, 61, 967–970. [Google Scholar] [CrossRef]
- Case, R.J.; Wang, Y.; Franzblau, S.G.; Soejarto, D.D.; Matainaho, L.; Piskaut, P.; Pauli, G.F. Advanced applications of counter-current chromatography in the isolation of anti-tuberculosis constituents from Dracaena angustifolia. J. Chromatogr. A 2007, 1151, 169–174. [Google Scholar] [CrossRef]
- Tran, Q.L.; Tezuka, Y.; Banskota, A.H.; Tran, Q.K.; Saiki, I.; Kadota, S. New spirostanol steroids and steroidal saponins from roots and rhizomes of Dracaena angustifolia and their antiproliferative activity. J. Nat. Prod. 2001, 64, 1127–1132. [Google Scholar] [CrossRef]
- Banskota, A.H.; Tezuka, Y.; Le Tran, Q.; Kadota, S. Chemical constituents and biological activities of Vietnamese medicinal plants. Curr. Top. Med. Chem. 2003, 3, 227–248. [Google Scholar] [CrossRef]
- Kravets, S.D.; Vollerner, Y.S; Gorovits, M.B.; Shashkov, A.S.; Abubakirov, N.K. Steroids of the spirostan and furostan series from plants of the genus Allium. XXI. Structure of alliospiroside a and alliofuroside a from Allium cepa. Chem. Nat. Compounds 1986, 22, 174–181. [Google Scholar]
- Yang, S.C.; Chung, P.J.; Ho, C.M.; Kuo, C.Y.; Hung, M.F.; Huang, Y.T.; Chang, W.Y.; Chang, Y.W.; Chan, K.H.; Hwang, T.L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013, 190, 6511–6519. [Google Scholar] [CrossRef]
- Yu, H.P.; Hsieh, P.W.; Chang, Y.J.; Chung, P.J.; Kuo, L.M.; Hwang, T.L. 2-(2-Fluorobenzamido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Bunsawansong, P.; Morris, G.A. Dependence of the 1H-NMR chemical shifts of ring F resonances on the orientation of the 27-methyl group of spirostane-type steroidal sapogenins. Phytochemistry 1998, 47, 255–257. [Google Scholar] [CrossRef]
- Tobari, A.; Teshima, M.; Koyanagi, J.; Kawase, M.; Miyamae, H.; Yoza, K.; Takasaki, A.; Nagamura, Y.; Saito, S. Spirostanols obtained by cyclization of pseudosaponin derivatives and comparison of anti-platelet agglutination activities of spirostanol glycosides. Eur. J. Med. Chem. 2000, 35, 511–527. [Google Scholar] [CrossRef]
- Huang, H.C.; Tsai, W.J.; Liaw, C.C.; Wu, S.H.; Wu, Y.C.; Kuo, Y.H. Anti-platelet aggregation triterpene saponins from the galls of Sapindus mukorossi. Chem. Pharm. Bull. 2007, 55, 1412–1415. [Google Scholar] [CrossRef]
- Lopes, J.F.; Gaspar, E.M. Simultaneous chromatographic separation of enantiomers, anomers and structural isomers of some biologically relevant monosaccharides. J. Chromatogr. A 2008, 1188, 34–42. [Google Scholar]
- Yen, C.T.; Lee, C.L.; Chang, F.R.; Hwang, T.L.; Yen, H.F.; Chen, C.J.; Chen, S.L.; Wu, Y.C. Indiosides G-K: Steroidal glycosides with cytotoxic and anti-inflammatory activities from Solanum violaceum. J. Nat. Prod. 2012, 75, 636–643. [Google Scholar] [CrossRef]
- Tsai, I.L.; Jeng, Y.F.; Jayaprasasam, B.; Chen, I.S. Cytotoxic constituents from the leaves of Litsea akoensis. Chin. Pharm. J. 2001, 53, 291–301. [Google Scholar]
- Hsieh, T.J.; Chang, F.R.; Chia, Y.C.; Chen, C.Y.; Chiu, H.F.; Wu, Y.C. Cytotoxic constituents of the fruits of Cananga odorata. J. Nat. Prod. 2001, 64, 616–619. [Google Scholar] [CrossRef]
- Talapatra, S.K.; Mukhopadhyay, S.K.; Talapatra, B. Minor coumarins of Boenninghausenia albiflora. Phytochemistry 1975, 14, 836–837. [Google Scholar]
- Zhang, H.; Conte, M.M.; Huang, X.C.; Khalil, Z.; Capon, R.J. A search for bace inhibitors reveals new biosynthetically related pyrrolidones, furanones and pyrroles from a southern australian marine sponge, Ianthella sp. Org. Biomol. Chem. 2012, 10, 2656–2663. [Google Scholar] [CrossRef]
- Leu, Y.L.; Chan, Y.Y.; Hsu, M.Y.; Chen, I.S.; Wu, T.S. The fresh stems and roots of Aristolochia foveolata MERR. (Aristolochia kaoi Liu and Lai) (Aristolochiaceae). J. Chin. Chem. Soc. 1998, 45, 539–542. [Google Scholar]
- Chen, C.Y.; Chang, F.R.; Teng, C.M.; Wu, Y.C. Cheritamine, A new n-fatty acyl tryptamine and other constituents from the stems of Annona cherimola. J. Chin. Chem. Soc. 1999, 46, 77–86. [Google Scholar]
- Wolfgang, A.; Charles, K.B. Synthesis of 11-hydroxy-2,3,9,10-tetramethoxy-5,6,13a-tetrahydro-8H-dibenzo[a,g]quinolizine. Structure of stepharotine. J. Org. Chem. 1969, 34, 1349–1352. [Google Scholar] [CrossRef]
- Wu, Y.C.; Sureshbabu, M.; Fang, Y.C.; Wu, Y.H.; Lan, Y.H.; Chang, F.R.; Chang, Y.W.; Hwang, T.L. Potent inhibition of human neutrophil activations by bractelactone, a novel chalcone from Fissistigma bracteolatum. Toxicol Appl. Pharmacol. 2013, 266, 399–407. [Google Scholar] [CrossRef]
- Hwang, T.L.; Su, Y.C.; Chang, H.L.; Leu, Y.L.; Chung, P.J.; Kuo, L.M.; Chang, Y.J. Suppression of superoxide anion and elastase release by C18 unsaturated fatty acids in human neutrophils. J. Lipid Res. 2009, 50, 1395–1408. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1-10 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, H.-C.; Lin, M.-K.; Hwang, S.-Y.; Hwang, T.-L.; Kuo, Y.-H.; Chang, C.-I.; Ou, C.-Y.; Kuo, Y.-H. Two Anti-inflammatory Steroidal Saponins from Dracaena angustifolia Roxb. Molecules 2013, 18, 8752-8763. https://doi.org/10.3390/molecules18088752
Huang H-C, Lin M-K, Hwang S-Y, Hwang T-L, Kuo Y-H, Chang C-I, Ou C-Y, Kuo Y-H. Two Anti-inflammatory Steroidal Saponins from Dracaena angustifolia Roxb. Molecules. 2013; 18(8):8752-8763. https://doi.org/10.3390/molecules18088752
Chicago/Turabian StyleHuang, Hui-Chi, Ming-Kuem Lin, Syh-Yuan Hwang, Tsong-Long Hwang, Yao-Haur Kuo, Chi-I Chang, Chung-Yi Ou, and Yueh-Hsiung Kuo. 2013. "Two Anti-inflammatory Steroidal Saponins from Dracaena angustifolia Roxb." Molecules 18, no. 8: 8752-8763. https://doi.org/10.3390/molecules18088752





