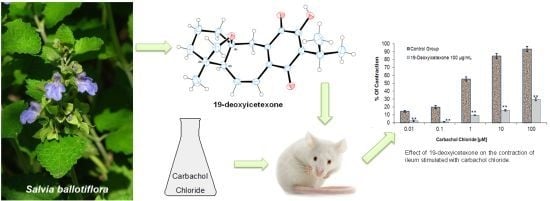
Antidiarrheal Activity of 19-Deoxyicetexone Isolated from Salvia ballotiflora Benth in Mice and Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Analyses
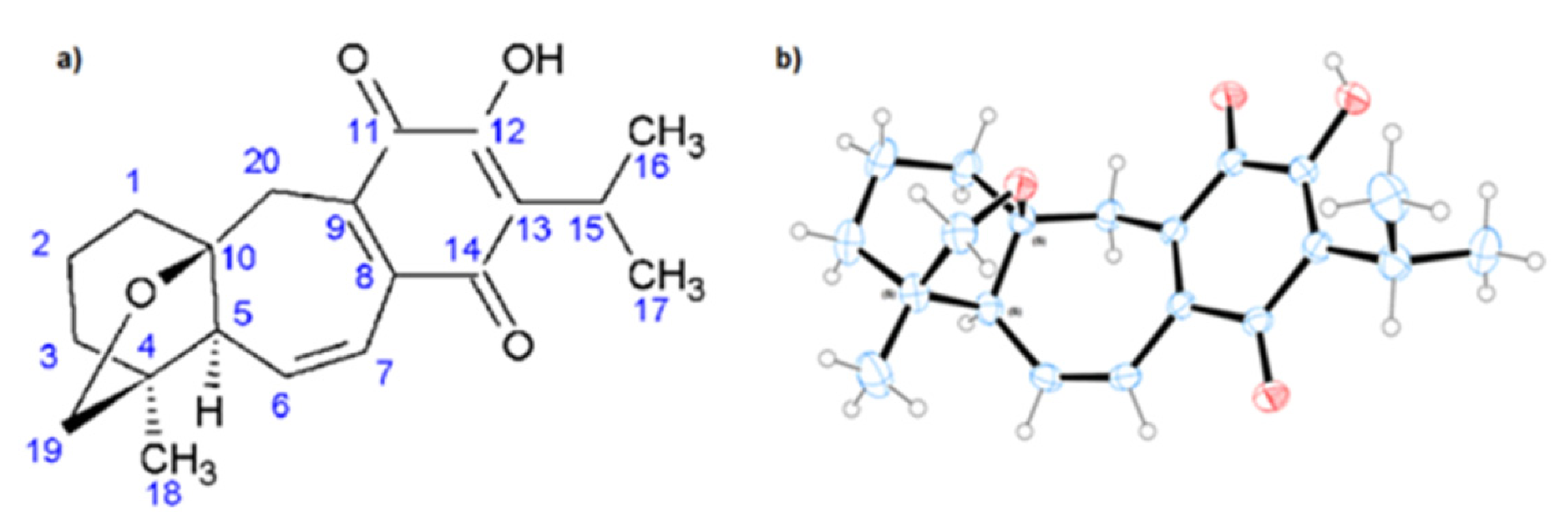
 264 and 317 nm (as opposed to mp 228–230 °C, [α]20 = +95 and the UV
264 and 317 nm (as opposed to mp 228–230 °C, [α]20 = +95 and the UV  211, 290, and 318 nm as previously reported). In addition, we add to the scientific record the complete 1H-NMR assignment of 19-deoxyicetexone (Table 1).
211, 290, and 318 nm as previously reported). In addition, we add to the scientific record the complete 1H-NMR assignment of 19-deoxyicetexone (Table 1).| Position | δ13C (ppm) | δ1H (ppm) | Position | δ13C (ppm) | δ1H (ppm) |
|---|---|---|---|---|---|
| 1 | 39.88 (39.8) * | 1.76 (m), 1.70 (m) | 12 | 150.75 (150.7) * | - |
| 2 | 20.19 (20.2) * | 1.89 (m), 1.68 (m) | 13 | 124.48 (124.4) * | - |
| 3 | 39.19 (39.1) * | 1.57(m), 1.50 (tdd) | 14 | 186.54 (186.5) * | - |
| 4 | 43.90 (43.9) * | - | 15 | 24.30 (24.3) * | 3.22 (hept) |
| 5 | 59.30 (59.3) * | 2.31 dd | 16 | 19.92 (19.9) * | 1.24 (d) |
| 6 | 141.82 (141.8) * | 6.51 dd | 17 | 19.88 (19.9) * | 1.23 (d) |
| 7 | 124.13 (124.1) * | 6.81 dd | 18 | 20.03 (19.5) * | 1.04 (s) |
| 8 | 135.63 (135.5) * | - | 19 | 77.72 (77.6) * | 3.70 (d), 3.50 (dd) |
| 9 | 139.95 (138.6) * | - | 20 | 33.23 (33.2) * | 2.98 (d), 2.59 (d) |
| 10 | 92.91 (92.9) * | - | OH | - | 7.17 (s) |
| 11 | 183.31 (183.3) * | - |
2.2. Pharmacological Study of 19-Deoxyicetexone
2.2.1. Acute Toxicity
2.2.2. Antidiarrheal Activity
| Treatment | Doses mg/kg | Percentage of inhibition |
|---|---|---|
| Vehicle | 0.1 mL | 0.0 |
| 19-Deoxyicetexone | 6.25 | 30.9 ± 7.2 ns |
| 12.5 | 61.8 ± 5.5 * | |
| 25 | 89.6 ± 1.7 ** | |
| Loperamide | 2.5 | 81.5 ± 4.5 ** |
| Cathartic agent | Treatment | Doses mg/kg | Percentage of inhibition |
|---|---|---|---|
| Arachidonic acid | Vehicle | 0.1 mL | 0.0 |
| 19-Deoxyicetexone | 25 | 75.0 ± 2.3 ** | |
| Loperamide | 2.5 | 77.8 ± 4.5 ** | |
| Prostaglandins E2 | Vehicle | 0.1 mL | 0.0 |
| 19-Deoxyicetexone | 25 | 22.2 ± 9.1 ns | |
| Loperamide | 2.5 | 69.4 ± 2.3** |
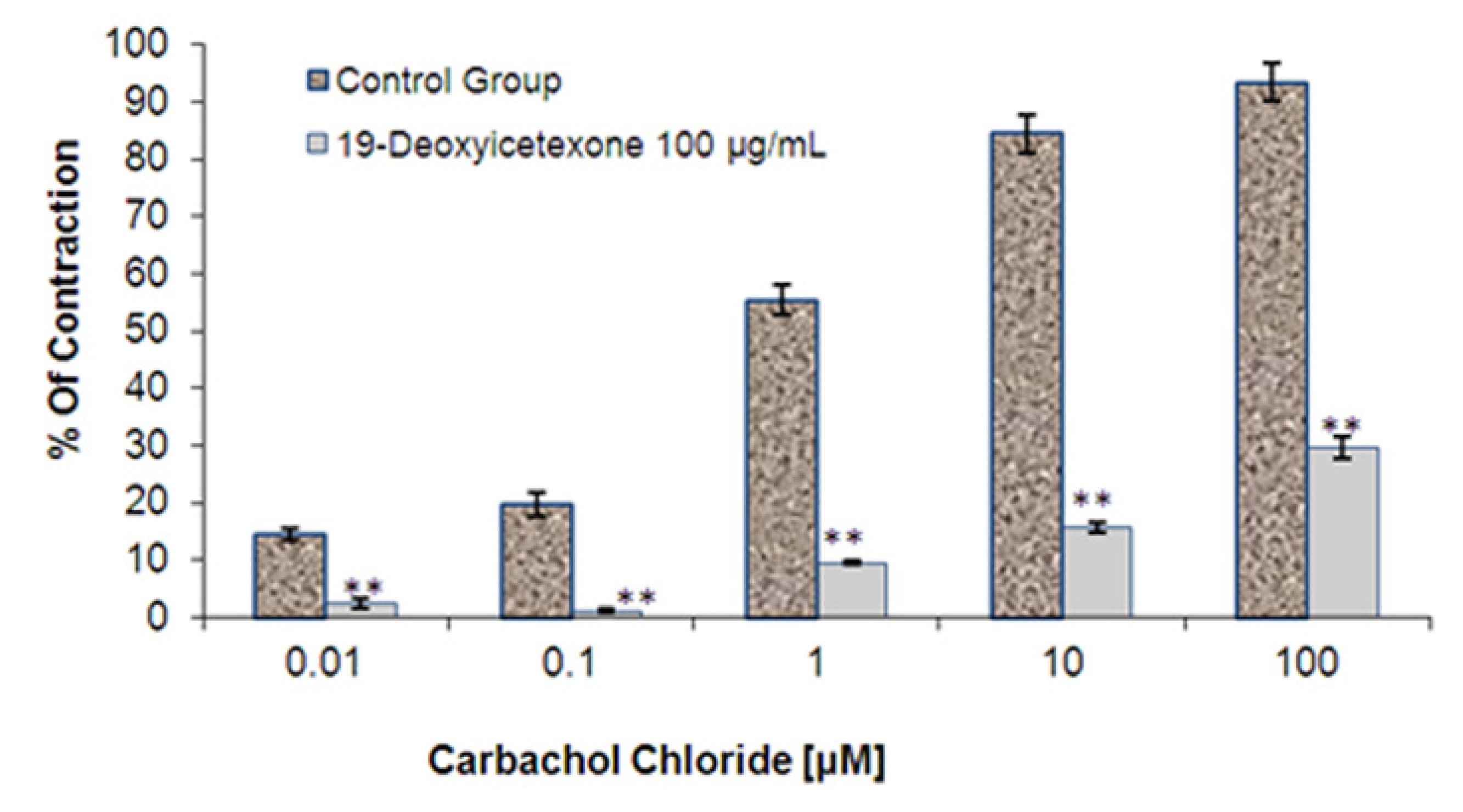
2.2.3. Ileum Contractions
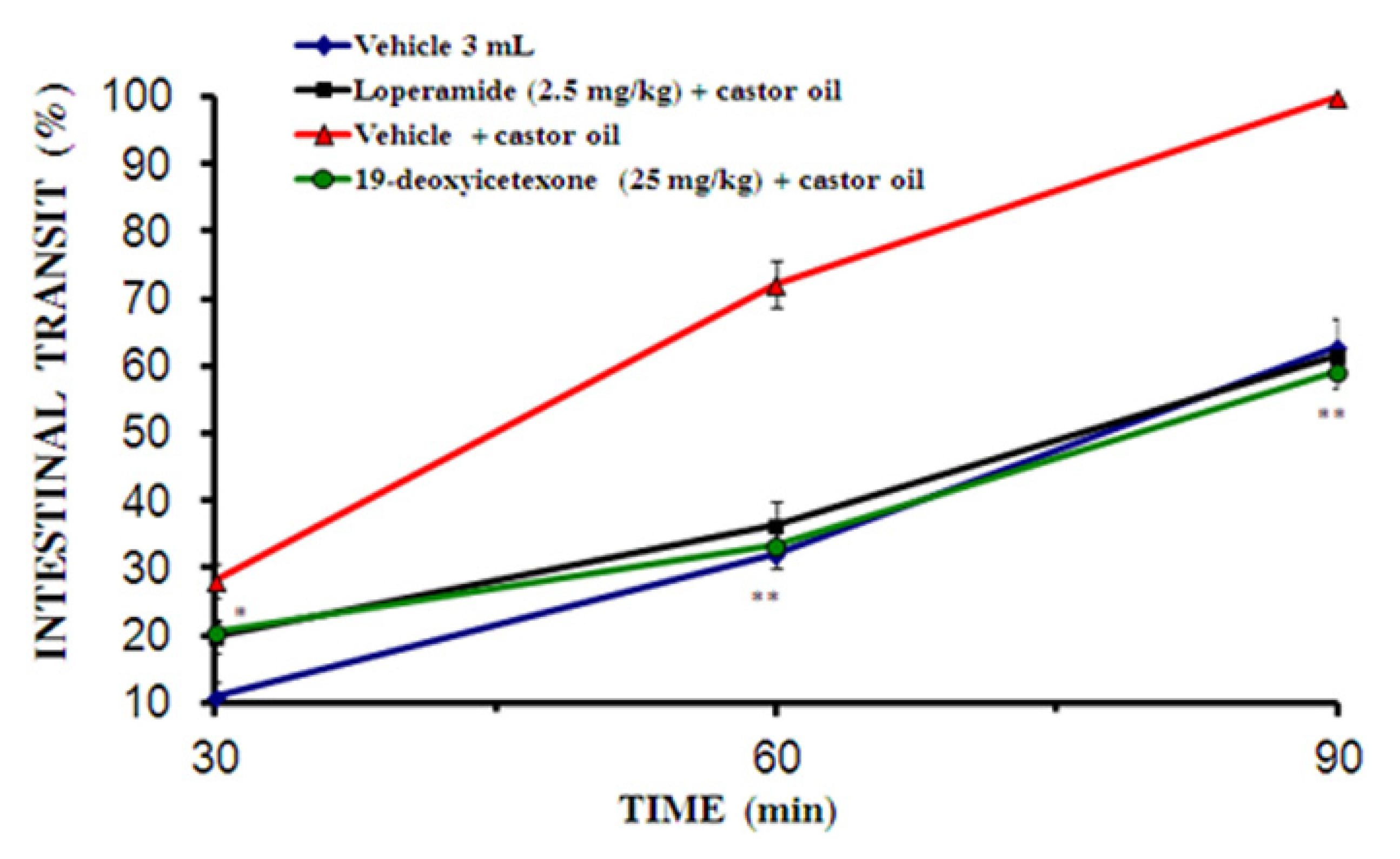
2.2.4. Small Intestinal Transit
2.2.5. Castor Oil-Induced Enteropooling
| Treatment | Dose (mg/kg) | Wt of intestinal content (g) | % Inhibition |
|---|---|---|---|
| Control (vehicle) | 2 mL | 0.5 ± 0.3 | |
| Castor oil | 2 mL | 3.0 ± 0.06 | |
| 19-Deoxyicetexone | 25 | 1.4 ± 0.2 * | 53.3 |
| Loperamide | 2.5 | 1.2 ± 0.3 * | 60.0 |
3. Experimental
3.1. Plant Material
3.2. Extraction and Isolation of 19-Deoxyicetexone
3.3. Structural Analysis of 19-Deoxyicetexone
3.4. Antidiarrheal Activity
3.4.1. Animals
3.4.2. Evaluation of Antidiarrheal Activity
3.4.3. Evaluation of Antidiarrheal Activity in AA- and PGE2-Induced Diarrhea
3.4.4. Evaluation of the Effects of 19-Deoxyicetexone on Ileum Contractions
3.4.5. Small Intestinal Transit
3.4.6. Castor Oil-Induced Enteropooling
3.4.7. Acute Toxicity
3.4.8. Statistical Analyses
4. Conclusions
Conflicts of Interest
References
- World Health Organization, Water and Sanitation; WHO: Geneva, Switzerland, 1996; Volume 12.
- Syder, J.D.; Merson, M.H. The magnitude of the global problems of acute diarrhoeal disease a review of active surveillance data. Bull. World Health Organ. 1982, 60, 605–613. [Google Scholar]
- Hardman, J.G.; Limbird, L.E. Goodman and Gilman’s: The Pharmacological Basis of Therapeutics; McGraw Hill: New York, NY, USA, 2010; pp. 914–931. [Google Scholar]
- Kumar, S.; Garg, V.K.; Kumar, N.; Sharma, P.K.; Chaudhary, S.; Upadhyay, A. Pharmacognostical studies on the leaves of Ziziphus nummularia (Burm. F.). Eur. J. Exper. Biol. 2011, 1, 77–83. [Google Scholar]
- Beverly, C.D.; Sudarsanam, G. Ethnomedicinal plant knowledge and practice of people of Javadhu hills in Tamilnadu. Asian Pac. J. Trop. Biomed. 2011, 1, S79–S81. [Google Scholar] [CrossRef]
- Wendel, G.H.; María, A.O.; Guzmán, J.A.; Giordano, O.; Pelzer, L.E. Antidiarrheal activity of dehydroleucodiene isolated from Artemisia douglasiana. Fitoterapia 2008, 79, 1–5. [Google Scholar] [CrossRef]
- Biblioteca digital de la Medicina Tradicional Mexicana. Available online: http://www.medicinatradicionalmexicana.unam.mx (accessed on 3 March 2013).
- Esquivel, B.; Calderón, J.S.; Flores, E.; Sánchez, A.A.; Rosas, R. Abietane and icetexane diterpenoids from Salvia ballotaeflora and Salvia axillaris. Phytochemistry 1997, 46, 531–534. [Google Scholar] [CrossRef]
- Cappasso, F.; Mascolo, N.; Izzo, A.A.; Gaginella, T.S. Dissociation of castor oil-induced diarrhea and intestinal mucosal injury in rat: Effect of NG nitro-L-arginine methylester. Br. J. Pharm. 1994, 113, 1127–1130. [Google Scholar] [CrossRef]
- Capasso, F.; Tavares, I.A.; Bennet, A. PAF formation by human gastrointestinal mucosa/submucosa in vitro: Release by ricinoleic acid, and inhibition by 5-aminosalicylic acid. J. Pharm. Pharmacol. 1992, 44, 771–772. [Google Scholar] [CrossRef]
- Mascolo, N.; Izzo, A.A.; Gaginella, T.S.; Capasso, F. Relationship between nitric oxide and platelet activating factor in castor oil-induced mucosal injury in the rat duodenum. Naunyn Schmiedebergs Arch. Pharmacol. 1996, 353, 680–684. [Google Scholar] [CrossRef]
- Uchida, M.; Yoshida, K.; Kato, Y.; Matsuede, K.; Shode, R.; Muraoka, A.; Yemato, S. Involvement of nitric oxide from nerves on diarrhea induced by castor oil in rats. Jpn. J. Pharmacol. 2000, 82, 168–170. [Google Scholar] [CrossRef]
- Galvez, J.; Zarzuelo, A.; Crespo, M.E.; Lorente, M.D.; Ocete, M.A.; Jimenez, J. Anti-diarrheic activity of Euphorbia hirta extract and isolation pf an active flavonoids constituents. Planta Medica 1993, 59, 333–336. [Google Scholar]
- Edwards, R.; Smock, A. Defective arachidonate release and PGE2 production in Giα2-deficient intestinal and colonic subepithelial myofibroblasts. Inflamm. Bowel Dis. 2006, 12, 153–155. [Google Scholar]
- Venegas, R.; Pérez, S.; Ferrer, R.; Moreno, J.J. Arachidonic acid cascade and epithelial barrier function during Caco-2 cell differentiation. J. Lipid Res. 2006, 47, 1416–1423. [Google Scholar] [CrossRef]
- Bao, X.; Liu, D.; Jin, Y.; Yang, Y. A facile synthesis for novel loperamide analogs as potential µ opioid receptors agonists. Molecules 2012, 17, 14288–14297. [Google Scholar]
- Kojima, Y.; Tsunoda, Y.; Owyang, C. Adenosine 39,59-cyclic monophosphate-stimulated Ca++ efflux and acetylcholine release in ileal myenteric plexus are mediated by N-type Ca++ channels: Inhibition by the kappa opioid receptor agonist. JPET 1997, 282, 403–409. [Google Scholar]
- Dharmsathaphorn, K.; Cohn, J.; Beuerlein, G. Multiple calcium-mediated effector mechanisms regulate chloride secretory responses in T84 cells. Am. J. Physiol. 1989, 256, C1224–C1230. [Google Scholar]
- Gaginella, T.S.; Phillips, S.F. Ricinoleic acid: Current view of ancient oil. Am. J. Dig. Dis. 1975, 20, 1171–1177. [Google Scholar] [CrossRef]
- The International Guiding Principles for Biomedical Research Involving Animals. The Council for International Organizations of Medical Sciences (CIOMS), 1985. Available online: http://cioms.ch/publications/guidelines/1985_texts_of_guidelines.htm/ (accessed on 17 July 2013).
- Awouters, F.; Niemegeers, C.J.S.; Lenaerts, F.M.; Jansen, P.A.J.J. Delay of castor oil diarrhoea in rats: A new way to evaluate inhibitors of Prostaglandin biosynthesis. J. Pharm. Pharmacol. 1978, 30, 41–45. [Google Scholar]
- Zavala, M.A. Estudio Químico-Farmacológico de Plantas con Actividad Antidiarreica; Tesis doctoral; Universidad Autónoma Metropolitana: Unidad Xochimilco, Coyoacán Ciudad de México, México, 1998. [Google Scholar]
- Melo, M.; Thomas, G.; Mukherjee, R. Antidiarrhoeal activity of bisnodihydrotoxiferine isolated from the root bark of Strychnos trinervis (Vell.) Mart. (Loganiaceae). J. Pharm. Pharmacol. 1988, 40, 79–82. [Google Scholar] [CrossRef]
- Estrada, S.; Gonzalez, D.; Castillo, P.; Aguirre, F.; Sánchez, J. Spasmolityc effect of Mentha pulegium L. involves ionic flux regulation in rat ileum strips. J. Smooth Muscle Res. 2010, 46, 107–117. [Google Scholar] [CrossRef]
- Vischer, P.; Casals-Stenzel, J. Influence of prostacyclin and indometacine on castor oil-induced gastrointestinal effects in rats. J. Pharm. Pharmacol. 1983, 35, 152–155. [Google Scholar] [CrossRef]
- Robert, A.; Nezamis, J.E.; Lancaster, C.; Hanchar, A.J.; Klepper, M.S. Enteropooling assay; a test for diarrhea produced by prostaglandins. Prostaglandins 1976, 11, 809–828. [Google Scholar]
- OECD, Guideline for Testing of Chemicals. Acute Oral Toxicity—Fixed Dose Procedure 420; OECD: Paris, France, 2001.
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pérez-Gutiérrez, S.; Zavala-Mendoza, D.; Hernández-Munive, A.; Mendoza-Martínez, Á.; Pérez-González, C.; Sánchez-Mendoza, E. Antidiarrheal Activity of 19-Deoxyicetexone Isolated from Salvia ballotiflora Benth in Mice and Rats. Molecules 2013, 18, 8895-8905. https://doi.org/10.3390/molecules18088895
Pérez-Gutiérrez S, Zavala-Mendoza D, Hernández-Munive A, Mendoza-Martínez Á, Pérez-González C, Sánchez-Mendoza E. Antidiarrheal Activity of 19-Deoxyicetexone Isolated from Salvia ballotiflora Benth in Mice and Rats. Molecules. 2013; 18(8):8895-8905. https://doi.org/10.3390/molecules18088895
Chicago/Turabian StylePérez-Gutiérrez, Salud, Daniel Zavala-Mendoza, Abigail Hernández-Munive, Ángel Mendoza-Martínez, Cuauhtemoc Pérez-González, and Ernesto Sánchez-Mendoza. 2013. "Antidiarrheal Activity of 19-Deoxyicetexone Isolated from Salvia ballotiflora Benth in Mice and Rats" Molecules 18, no. 8: 8895-8905. https://doi.org/10.3390/molecules18088895