Cytotoxic Activity of Ursolic Acid Derivatives Obtained by Isolation and Oxidative Derivatization
Abstract
:1. Introduction

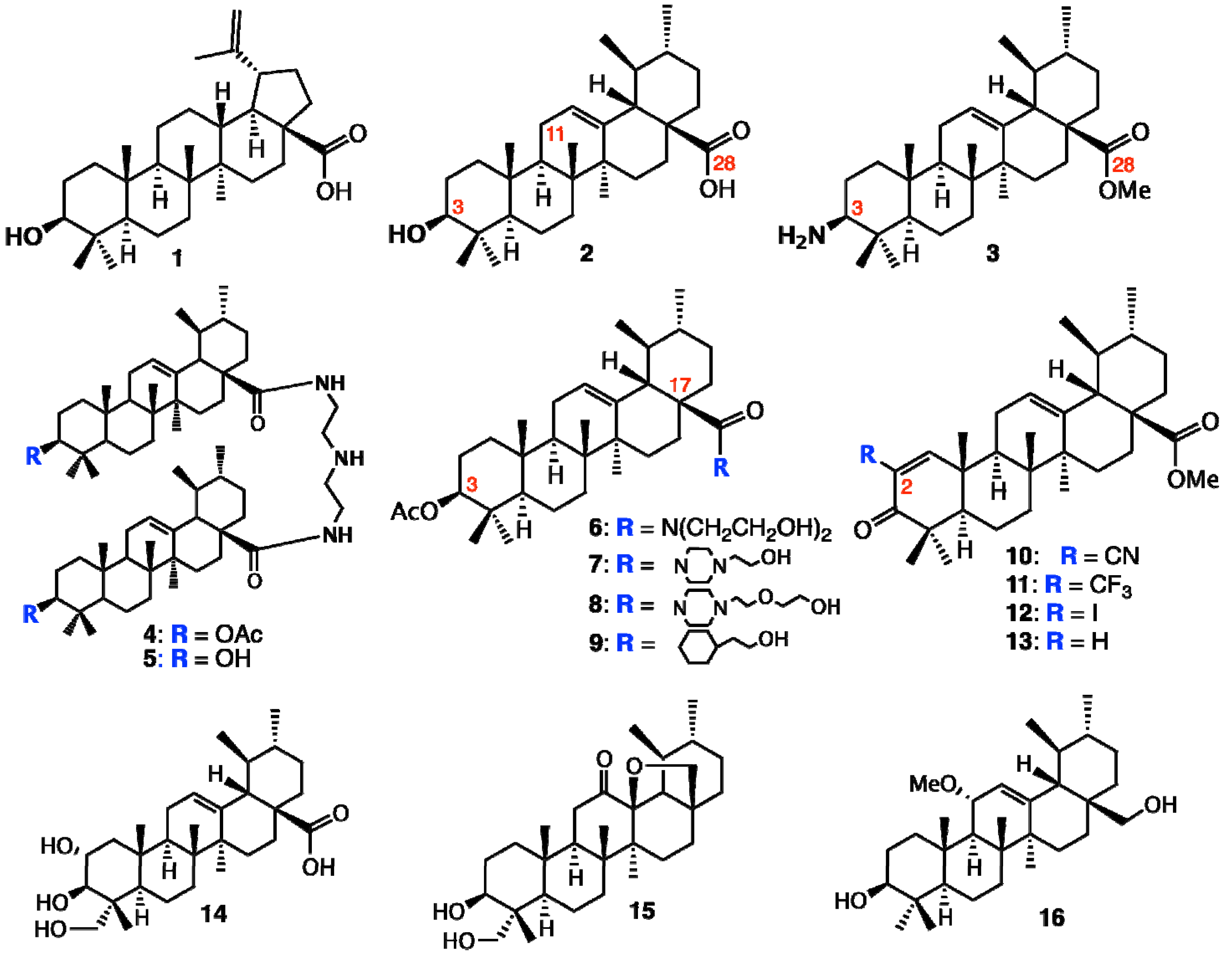
2. Ursane-Type Natural Triterpenes from Plants
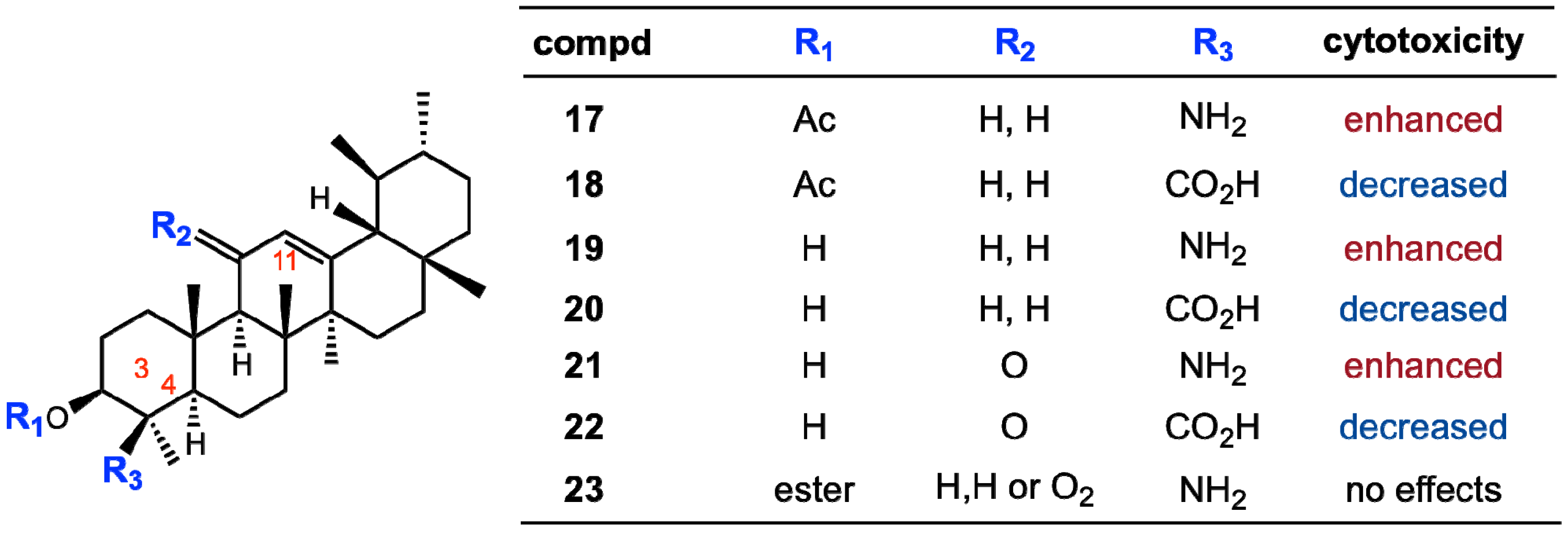
2.1. Isolation of Triterpene Natural Products from Leaves of Saurauja roxburghii
2.2. Structural Determination of Ursane-Type Triterpenes
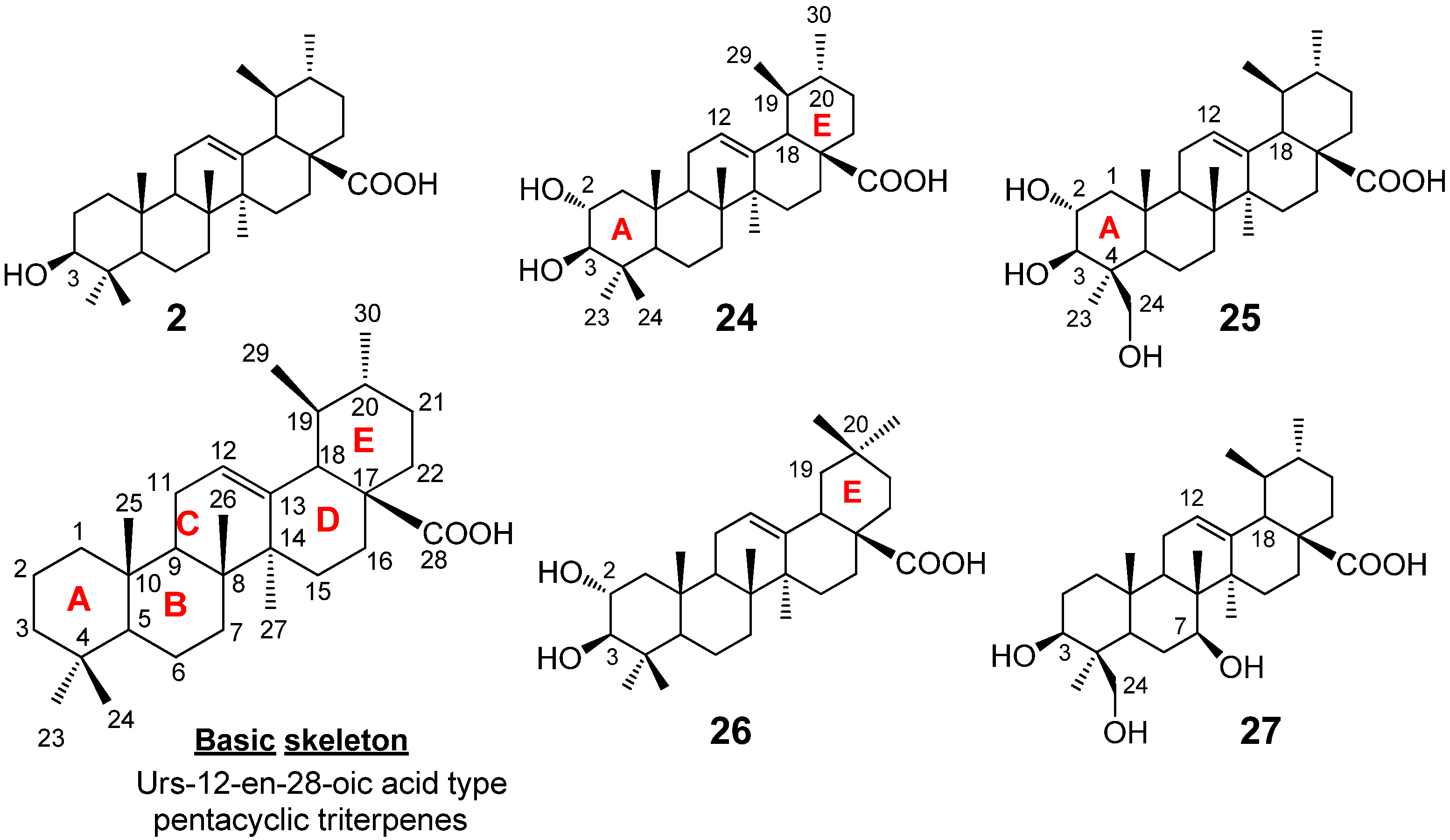
3. Biomimetic Oxidative Derivatization of Ursolic Acid
3.1. Development of Auxillary-Directed ‘Ru’-tetraphenylporphyrin Derivatives and Oxidative Derivatization of Ursolic Acid

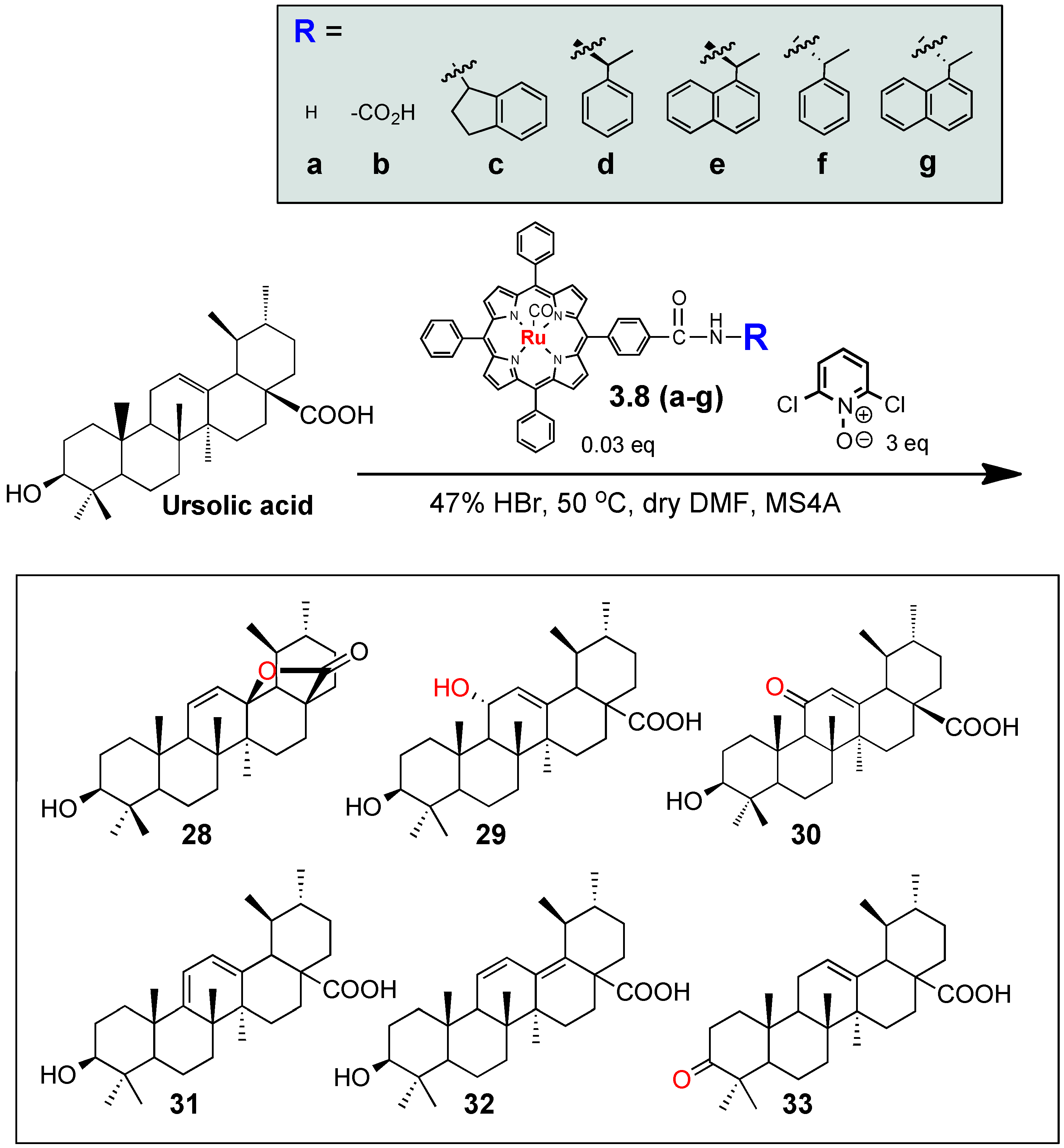
| Entry | Porphyrin catalyst | Product yields (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 2 (recovery) | 28 | 29 | 30 | 31 | 32 | 33 | ||
| 1 | 3.8a | 25 | 25 | 35 | 5 | ND | ND | ND |
| 2 | 3.8b | 35 | 35 | 4 | 8 | 10 | 8 | ND |
| 3 | 3.8c | 80 | ND | 5 | 5 | ND | ND | 10 |
| 4 | 3.8d | ND | ND | 70 | 5 | ND | ND | ND |
| 5 | 3.8e | 50 | ND | 30 | 10 | ND | ND | ND |
| 6 | 3.8f | 75 | ND | 5 | 5 | ND | ND | ND |
| 7 | 3.8g | 90 | ND | 10 | ND | ND | ND | ND |
4. Evaluation of Cytotoxic Activity towards Tumor Cell Lines
| Compound | A431 human epidermoid carcinoma | C6 rat glioma |
|---|---|---|
| 2 | − | + |
| 24 | + | + |
| 25 | − | − |
| 26 | − | − |
| 27 | − | − |
| 28 | + | + |
| 29 | − | − |
| 30 | − | − |
| 31 | − | − |
| 32 | − | − |
| 33 | − | − |
5. Conclusions
Conflicts of Interest
References and Notes
- Knowles, J.; Gromo, G. Target selection in drug discovery. Nat. Rev. Drug Discov. 2003, 2, 63–69. [Google Scholar] [CrossRef]
- Samuelsson, G. Drugs of Natural Origin: a Textbook of Pharmacognosy, 5th ed.; Swedish Pharmaceutical Press: Stockholm, Sweden, 2004; pp. 154–155. [Google Scholar]
- Balick, M.J.; Cox, P.A. Plants, People, and Culture: the Science of Ethnobotany, 1st ed.; Freeman and Co: Scientific American Library, New York, NY, USA, 1997; pp. 8–9. [Google Scholar]
- Kinghorn, A.D. Pharmacognosy in the 21st century. J. Pharm. Pharmacol. 2001, 53, 135–148. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. The influence of natural products upon drug discovery. Nat. Prod. Rep. 2000, 17, 215–234. [Google Scholar] [CrossRef]
- Butler, M.S. The role of natural product chemistry in drug discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of new drugs over the period 1981-2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef]
- van der Heijden, R.; Jacobs, D.I.; Snoeijer, W.; Hallard, D.; Verpoorte, R. The Catharanthus alkaloids: Pharmacognosy and biotechnology. Curr. Med. Chem. 2004, 11, 607–628. [Google Scholar] [CrossRef]
- Gordaliza, M.; Garcia, P.A.; Corral, J.M.; Castro, M.A.; Gomez-Zurita, M.A. Podophyllotoxin: Distribution, sources, applications and new cytotoxic derivatives. Toxicon 2004, 44, 441–459. [Google Scholar] [CrossRef]
- Wall, M.E.; Wani, M.C. Camptothecin and taxol: From discovery to clinic. J. Ethnopharmacol. 1996, 51, 239–253. [Google Scholar] [CrossRef]
- Oberlies, N.H.; Kroll, D.J. Camptothecin and taxol: Historic achievements in natural products research. J. Nat. Prod. 2004, 67, 129–135. [Google Scholar] [CrossRef]
- Cragg, G.M.; D.J. Newman, D.J. A tale of two tumor targets: Topoisomerase I and tubulin. The Wall and Wani contribution to cancer chemotherapy. J. Nat. Prod. 2004, 67, 232–244. [Google Scholar] [CrossRef]
- Ikeda, Y.; Murakami, A.; H. Ohigashi, H. Ursolic acid: An anti- and pro-inflammatory triterpenoid. Mol. Nutr. Food Res. 2008, 52, 26–42. [Google Scholar] [CrossRef]
- Pisha, E.; Chai, H.; Lee, I.S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.; Fong, H.H.; Kinghorn, A.D.; Brown, D.M. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef]
- Andersson, D.; Cheng, Y.; Duan, R.D. Ursolic acid inhibits the formation of aberrant crypt foci and affects colonic sphingomyelin hydrolyzing enzymes in azoxymethane-treated rats. J. Cancer Res. Clin. Oncol. 2008, 134, 101–107. [Google Scholar] [CrossRef]
- Bonaccorsi, I.; Altieri, F.; Sciamanna, I.; Oricchio, E.; Grillo, C.; Contartese, G.; Galati, E.M. Endogenous reverse transcriptase as a mediator of ursolic acid's antiproliferative and differentiating effects in human cancer cell lines. Cancer Lett. 2008, 263, 130–139. [Google Scholar] [CrossRef]
- Ma, C.-M.; Cai, S.-Q.; Cui, J.-R.; Wang, R.-Q.; Tu, P.-F.; Hattori, M.; Daneshtalab, M. The cytotoxic activity of ursolic acid derivatives. Eur. J. Med. Chem. 2005, 40, 582–589. [Google Scholar] [CrossRef]
- Shao, J.-W.; Dai, Y.-C.; Xue, J.-P.; Wang, Ji.-C.; Lin, F.-P.; Guo, Y.-H. In vitro and in vivo anticancer activity evaluation of ursolic acid derivatives. Eur. J. Med. Chem. 2011, 46, 2652–2661. [Google Scholar] [CrossRef]
- Chadalapaka, G.; Jutooru, I.; McAlees, A.; Stefanac, T.; Safe, S. Structure-dependent inhibition of bladder and pancreatic cancer cell growth by 2-substituted glycyrrhetinic and ursolic acid derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 2633–2639. [Google Scholar] [CrossRef]
- Park, B.C.; Paek, S.H.; Lee, Y.S.; Kim, S.J.; Lee, E.S.; Choi, H.G.; Yong, C.S.; Kim, J.A. Inhibitory effects of asiatic acid on 7, 12-dimethylbenz[〈]anthracene and 12-O-tetradecanoylphorbol 13-acetate-induced tumor promotion in mice. Biol. Pharm. Bull. 2007, 30, 176–179. [Google Scholar] [CrossRef]
- Chen, I.H.; Chang, F.R.; Wu, C.C.; Chen, S.L.; Hsieh, P.W.; Yen, H.F.; Du, Y.C.; Wu, Y.C. Cytotoxic triterpenoids from the leaves of Microtropis fokienensis. J. Nat. Prod. 2006, 69, 1543–1546. [Google Scholar] [CrossRef]
- Mazumder, K.; Swiu, E.R.O.; Nozaki, S.; Watanabe, Y.; Tanaka, K.; Fukase, K. Ursolic Acid Derivatives from Bangladeshi medicinal plant, Sarauja roxburghii: Isolation and cytotoxic activity against A431 and C6 glioma cell lines. Phytochem. Lett. 2011, 4, 287–291. [Google Scholar] [CrossRef]
- Tanaka, K.; Mazumder, K.; Siwu, E.R.O.; Nozaki, S.; Watanabe, Y.; Fukase, K. Auxiliary-directed oxidation of ursolic acid by “Ru”-porphyrins: Chemical modulation of cytotoxicity against tumor cell lines. Tetrahedron Lett. 2012, 53, 1756–1759. [Google Scholar] [CrossRef]
- Hsu, Y.-L.; Kuo, P.L.; Lin, L.T.; Lin, C.C. Asiatic acid, a triterpene, induces apoptosis and cell cycle arrest through activation of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways in human breast cancer cells. J. Phamacol. Exp. Ther. 2004, 313, 333–344. [Google Scholar] [CrossRef]
- Seo, S.; Tomita, Y.; Tori, K. Carbon-13 NMR spectra of urs-12enes and application of structural assignments of components of Isodon japonicus tissue cultures. Tetrahedron Lett. 1975, 16, 7–10. [Google Scholar] [CrossRef]
- Reher, G.; Budensisky, M. Triterpenoids from plants of Sanguisorbae. Photochemistry 1992, 31, 3909–3914. [Google Scholar] [CrossRef]
- Inada, A.; Kobayashi, M.; Murata, H.; Nakanishi, T. Two new triterpenoid glycosides from leaves of Ilex chinensis SIMS. Chem. Pharm. Bull. 1987, 35, 841–842. [Google Scholar] [CrossRef]
- Kojima, H.; Ogura, H. Configurational studies on hydroxyl groups at C-2, 3 and C-23 of oleanene and ursene-type trirerpenes by NMR spectroscopy. Phytochemistry 1989, 28, 1703–1989. [Google Scholar] [CrossRef]
- Bilia, A.R.; Mendezb, J.; Morellia, I. Phytochemical investigations of Licania genus. Flavonoids and triterpenoids from Licania carii. Pharm. Acta Helv. 1996, 71, 191–197. [Google Scholar] [CrossRef]
- Lee, C.K. Ursane triterpenoids from leaves of Melaleuca leucadendron. Phytochemistry 1998, 49, 1119–1122. [Google Scholar] [CrossRef]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; Lupiáñez, J.A.; Cascante, M. Maslinic acid, a natural triterpene from Olea europaea L., induces apoptosis in HT29 human colon-cancer cells via the mitochondrial apoptotic pathway. Cancer Lett. 2009, 273, 44–54. [Google Scholar] [CrossRef]
- Murahashi, S.-I.; Zhang, D. Ruthenium catalyzed biomimetic oxidation in organic synthesis inspired by cytochrome P-450. Chem. Soc. Rev. 2008, 37, 1490–1501. [Google Scholar] [CrossRef]
- P450-Mediated oxidation of ursolic acid derivatives, see: Sivakumar, G.; Vail, D.R.; Nair, V.; Bolivar, F.M.; Lay, J.O. Corosolic acid the future anti-diabetic drug? Biotechnol. J. 2009, 4, 1704–1711. [Google Scholar] [CrossRef]
- Patocka, J.J. Biologically active pentacyclic triterpenes and their current medicine signification. Appl. Biomed. 2003, 1, 7–12. [Google Scholar]
- Ma, C.M.; Nakamura, N.; Hattori, M.; Kakuda, H.; Qiao, J.C.; Yu, H.L.J. Inhibitory effects on HIV-1 protease of constituents from the wood of Xanthoceras sorbifolia. Nat. Prod. 2000, 63, 238–242. [Google Scholar] [CrossRef]
- Saraswat, B.; Visen, P.K.; Agarwal, D.P. Ursolic acid isolated from Eucalyptus tereticornis protects against ethanol toxicity in isolated rat hepatocytes. Phytother. Res. 2000, 14, 163–166. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Arenachalam, G.; Mandal, A.B.; Sur, T.K.; Mandal, S.C.; Bhattacharya, S.K. Antimicrobial and anti-inflammatory activity of folklore: Mallotus peltatus leaf extract. J. Ethnopharmacol. 2002, 82, 229–237. [Google Scholar] [CrossRef]
- Li, J.; Guo, W.J.; Yang, Q.Y. Effects of ursolic acid and oleanolic acid on human colon carcinoma cell line HCT15. World J. Gastroenterol. 2002, 8, 493–495. [Google Scholar]
- Anderson, D.; Liu, J.J.; Nilsson, A. Ursolic acid inhibits proliferation and stimulates apoptosis in HT29 cells following activation of alkaline sphingomyelinase. Anticancer Res. 2003, 23, 3317–3322. [Google Scholar]
- Choi, Y.H.; Baek, J.H.; Yoo, M.A.; Chung, H.Y.; Kim, N.D.; Kim, K.W. Induction of apoptosis by ursolic acid through activation of caspases and down-regulation of c-IAPs in human prostate epithelial cells. J. Oncol. 2000, 17, 565–570. [Google Scholar]
- Laszczyk, M.N. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009, 15, 1549–1560. [Google Scholar] [CrossRef]
- Breslow, R.; Baldwin, S.; Flechtner, T.; Kalicky, P.; Liu, S.; Washburn, W. Remote oxidation of steroids by photolysis of attached benzophenone groups. J. Am. Chem. Soc. 1973, 95, 3251–3262. [Google Scholar] [CrossRef]
- Das, S.; Incarvito, C.D.; Crabtree, R.H.; Brudvig, G.W. Molecular recognition in the selective oxygenation of saturated C-H bonds by a dimanganese catalyst. Science 2006, 312, 1941–1943. [Google Scholar] [CrossRef]
- Chen, M.S.; White, C.M. A Predictably Selective Aliphatic C-H Oxidation Reaction for Complex Molecule Synthesis. Science 2007, 318, 783–787. [Google Scholar] [CrossRef]
- Murahashi, S.-I. Development of biomimetic catalytic oxidation methods and non-salt methods using transition metal-based acid and base amphiphilic catalysts. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 242–253. [Google Scholar] [CrossRef]
- Representative example, see: Chen, K.; Baran, P.S. Total synthesis of eudesmane terpenes by site-selective C–H oxidations. Nature 2009, 459, 824–828. [Google Scholar] [CrossRef]
- Zhang, K.; Damaty, E.l.S.; Fasan, R. P450 fingerprinting method for rapid discovery of terpene hydroxylating P450 catalysts with diversified regioselectivity. J. Am. Chem. Soc. 2011, 133, 3242–3245. [Google Scholar] [CrossRef]
- Costas, M. Selective C-H oxidation catalyzed by metalloporphyrins. Coord. Chem. Rev. 2011, 255, 2912–2932. [Google Scholar] [CrossRef]
- Ogawa, S.; Wakatsuki, Y.; Makino, M.; Fujimoto, Y.; Yasukawa, K.; Kikuchi, T.; Ukiya, M.; Akihisa, T.; Iida, T. Oxyfunctionalization of unactivated C-H bonds in triterpenoids with tert-butylhydroperoxide catalyzed by meso-5,10,15,20-tetramesitylporphyrinate osmium(II) carbonyl complex. Chem. Phys. Lipids 2010, 163, 165–171. [Google Scholar] [CrossRef]
- Konoike, T.; Araki, Y.; Kanda, Y. A novel allylic hydroxylation of sterically hindered olefins by Fe-porphyrin-catalyzed mCPBA oxidation. Tetrahedron Lett. 1999, 40, 6971–6974. [Google Scholar] [CrossRef]
- Shingaki, T.; Miura, K.; Higuchi, T.; Hirobe, M.; Nagano, T. Regio- and stereo-selective oxidation of steroids using 2,6-dichloropyridine N-oxide catalysed by rutheniumporphyrins. Chem. Commun. 1997, 861–862. [Google Scholar]
- Che, C.-M.; Huang, J.-S. Metalloporphyrin-based oxidation systems: From biomimetic reactions to application in organic synthesis. Chem. Commun. 2009, 27, 3996–4015. [Google Scholar]
- Rillema, D.P.; Nagle, J.K.; Barringer, L.F.; Meyer, T.J. Redox properties of metalloporphyrin excited states, lifetimes, and related properties of a series of para-substituted tetraphenylporphyrin carbonyl complexes of ruthenium(II). J. Am. Chem. Soc. 1981, 103, 56–62. [Google Scholar] [CrossRef]
- Tkachev, A.V.; Denisov, A.Y.; Gatilov, Y.V.; Bagryanskaya, I.Y.; Shevtsov, S.A.; Rybalova, T.V. Acetic acid oxidation of ursolic acid and related compounds. Tetrahedron 1994, 50, 11459–11488. [Google Scholar] [CrossRef]
- Pereira, S.I.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D.; Silva, A.M.S. Chemical composition of the epicuticular wax from the fruits of Eucalyptus globulus. Phytochem. Anal. 2005, 16, 364–369. [Google Scholar] [CrossRef]
- Syamasundar, K.V.; Mallavarapu, G.R.; Krishna, M. Triterpenoids of the resin of Bursera delpechiana. Phytochemistry 1991, 30, 362–363. [Google Scholar] [CrossRef]
- Luis, J.G.; Andres, L.S. New ursane type triterpenes from Salvia mellifera greene. Nat. Prod. Lett. 1999, 13, 187–194. [Google Scholar] [CrossRef]
- Li, Y.; Matsuda, H.; Yoshikawa, M. Effects of oleanolic acid glycosides on gastrointestinal transit and ileus in mice. Bioorg. Med. Chem. 1999, 7, 1201–1205. [Google Scholar] [CrossRef]
- Wen, X.; Xia, J.; Cheng, K.; Zhang, L.; Zhang, P.; Liu, J.; Zhang, L.; Ni, P.; Sun, H. Pentacyclic triterpenes. Part 5: Synthesis and SAR study of corosolic acid derivatives as inhibitors of glycogen phosphorylases. Bioorg. Med. Chem. Lett. 2007, 17, 5777–5782. [Google Scholar] [CrossRef]
- Lee, Y.S.; Jin, D.Q.; Kwon, E.J.; Park, S.H.; Lee, E.S.; Jeong, T.C.; Nam, D.H.; Huh, K.; Kim, J.A. Asiatic acid, a triterpene, induces apoptosis through intracellular Ca2+ release and enhanced expression of p53 in HepG2 human hepatoma cells. Cancer Lett. 2002, 186, 83–91. [Google Scholar] [CrossRef]
- Cho, C.W.; Choi, D.S.; Cardone, M.H.; Kim, C.W.; Sinskey, A.J.; Rha, C. Glioblastoma cell death induced by asiatic acid. Cell Biol. Toxicol. 2006, 22, 393–408. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mazumder, K.; Tanaka, K.; Fukase, K. Cytotoxic Activity of Ursolic Acid Derivatives Obtained by Isolation and Oxidative Derivatization. Molecules 2013, 18, 8929-8944. https://doi.org/10.3390/molecules18088929
Mazumder K, Tanaka K, Fukase K. Cytotoxic Activity of Ursolic Acid Derivatives Obtained by Isolation and Oxidative Derivatization. Molecules. 2013; 18(8):8929-8944. https://doi.org/10.3390/molecules18088929
Chicago/Turabian StyleMazumder, Kishor, Katsunori Tanaka, and Koichi Fukase. 2013. "Cytotoxic Activity of Ursolic Acid Derivatives Obtained by Isolation and Oxidative Derivatization" Molecules 18, no. 8: 8929-8944. https://doi.org/10.3390/molecules18088929




