Inhibitors of Urokinase Type Plasminogen Activator and Cytostatic Activity from Crude Plants Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening of Medicinal Plants for uPA Inhibitory Activity
| Plant species | Origin | uPA inhibitory activity * |
|---|---|---|
| Amyris elemifera | Florida, Bahamas, Puerto Rico, West Indies, Central America. | + |
| Bidens alba L. | Native to South America. Pan tropical weed | – |
| Cananga odorata | Native to southern Asia and Philippines | + |
| Capparis cynophallophora | Florida, Bahamas, Puerto Rico, West Indies, Central America. | – |
| Colubrina elliptica | Puerto Rico, Florida, Bahamas, West Indies, Central Mexico toVenezuela | – |
| Croton lucidus | Native to PR, Bahamas, Greater Antilles, and Grand Cayman. | + |
| Erythroxylum areolatum | Bahamas, Greater Antilles, and South America | + |
| Exostema caribaeum | Florida, Bahamas, West Indies, Mexico, Central America, NE coast South America | – |
| Guaiacum officinale | Continental Tropical America, PR, West Indies | – |
| Kalanchoe pinnata | Native to Madagascar | – |
| Lantana camara | Pantropical | + |
| Plumeria alba L. | Central America and the Caribbean | – |
| Tabebuia heterophylla | Puerto Rico, Hispaniola, Virgin Islands, and Lesser Antilles. | + |
| Vanilla claviculata | Puerto Rico and Greater Antilles | – |
| Plant name | Partitions from crude methanol plant extracts * | |||
|---|---|---|---|---|
| Petroleum ether | Chloroform | Ethyl acetate | n-Butanol | |
| Amyris elemifera | – | – | + | – |
| Cananga odorata | – | – | + | – |
| Croton lucidus | + | + | + | + |
| Erythroxylum areolatum | – | – | – | + |
| Lantana camara | – | + | – | – |
| Tabebuia heterophylla | – | – | + | – |
2.2. uPA Inhibitory Activity of C. lucidus Extracts

| Extract | IC50 (µg/mL) |
|---|---|
| Crude methanol | 13.52 |
| Chloroform partition | 3.52 |
| n-Butanol partition | 26.51 |
| Ethyl acetate partition | 41.70 |
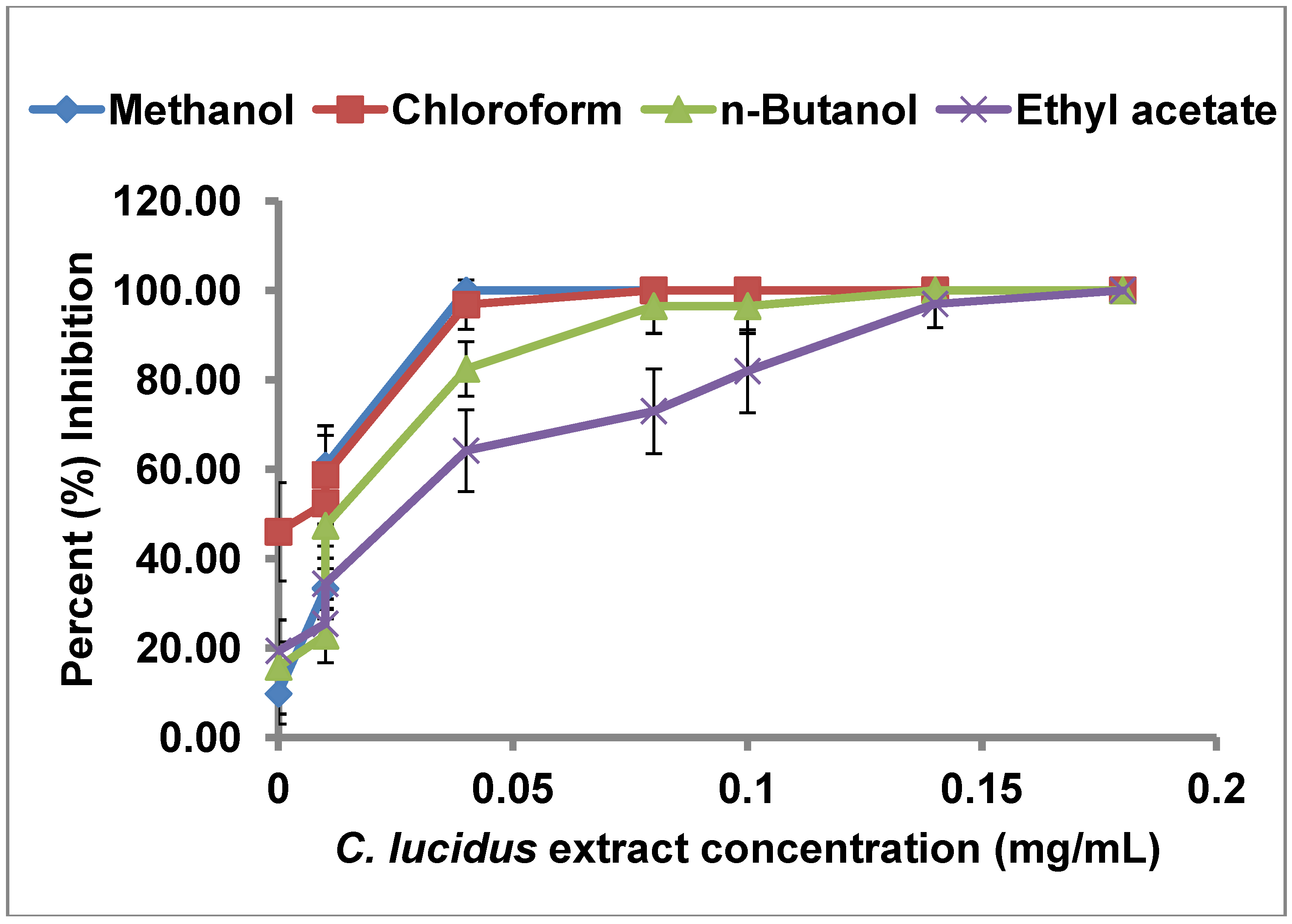
2.3. Selectivity of uPA Inhibitory Activity of C. lucidus Extracts
| Percent (%) Inhibition | |||
|---|---|---|---|
| uPA | tPA | Plasmin | |
| Crude methanol extract | 57.0 ± 2.2 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Chloroform partition | 74.0 ± 2.6 | 19.0 ± 0.8 | 14.0 ± 0.9 |
| n-Butanol partition | 40.0 ± 1.5 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Ethyl acetate partition | 24.0 ± 3.1 | 0.0 ± 0.0 | 32.0 ± 1.7 |
2.4. Effect of C. lucidus Extracts on Cell Viability
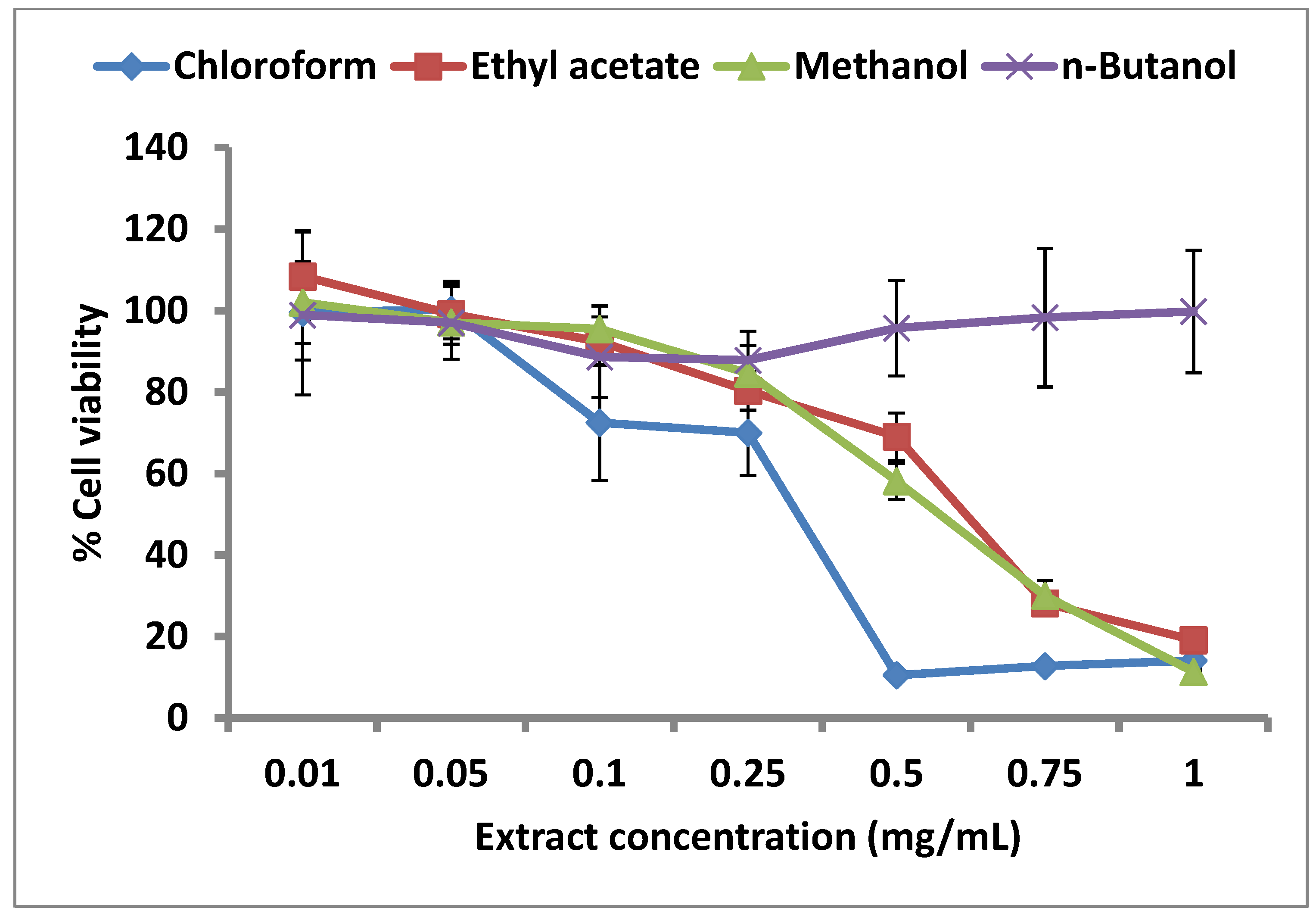
3. Experimental
3.1. Materials
3.2. Collection of Plant Materials
3.3. Preparation of Extracts
3.4. Bioassay of uPA-Inhibitory Activity of Extracts
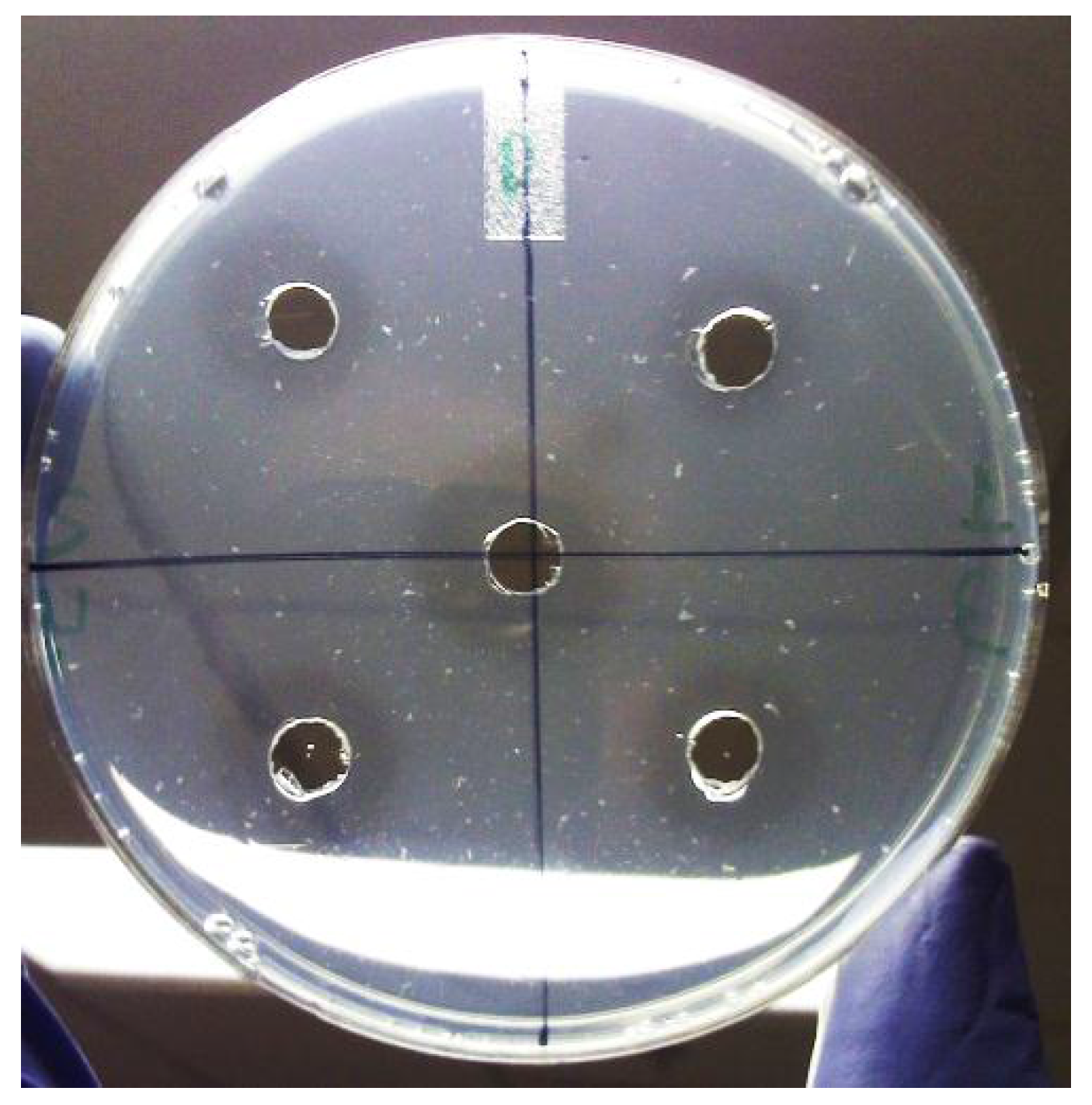
3.5. Determination of IC50
3.6. Bioassay of uPA Selectivity of Extracts
3.6.1. Inhibition of tPA
3.6.2. Inhibition of Plasmin
3.7. Cell Culture Assays

4. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Tang, L.; Han, X. The urokinase plasminogen activator system in breast cancer invasion and metastasis. Biomed. Pharmacother. 2013, 67, 179–182. [Google Scholar] [CrossRef]
- Roodman, G.D. Genes associate with abnormal bone cell activity in bone metastasis. Cancer Metastasis Rev. 2012, 31, 569–578. [Google Scholar] [CrossRef]
- Baldini, E.; Sorrenti, S.; D’Armiento, E.; di Matteo, F.M.; Catania, A.; Ulisse, S. The urokinase plasminogen activating system in thyroid cancer: Clinical implications. G. Chir. 2012, 33, 305–310. [Google Scholar]
- Hseu, Y.C.; Wu, C.R.; Chang, H.W.; Kumar, K.J.; Lin, M.K.; Chen, C.S.; Cho, H.J.; Huang, C.Y.; Lee, H.Z.; Hsieh, W.T.; et al. Inhibitory effects of Physalis angulata on tumor metastasis and angiogenesis. J. Ethnopharmacol. 2011, 135, 762–771. [Google Scholar] [CrossRef]
- Dunbar, S.D.; Ornstein, D.L.; Zacharski, L.R. Cancer treatment with inhibitors of urokinase-type plasminogen activator and plasmin. Expert Opin. Investig. Drugs 2000, 9, 2085–2092. [Google Scholar] [CrossRef]
- Ishii, K.; Usui, S.; Sugimura, Y.; Yamamoto, H.; Yoshikawa, K.; Hiran, K. Extract from Serenoa repens suppresses the invasion activity of human urological cancer cells by inhibiting urokinase-type plasminogen activator. Biol. Pharm. Bull. 2001, 24, 188–190. [Google Scholar] [CrossRef]
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006, 66, 2500–2505. [Google Scholar] [CrossRef]
- Schweinitz, A.; Steinmetzer, T.; Banke, I.J.; Arlt, M.J.; Sturzebecher, A.; Schuster, O.; Geissler, A.; Giersiefen, H.; Zeslawska, E.; Jacob, U.; et al. Design of novel and selective inhibitors of urokinase-type plasminogen activator with improved pharmacokinetic properties for use as antimetastatic agents. J. Biol. Chem. 2004, 279, 33613–33622. [Google Scholar] [CrossRef]
- Choong, P.F.; Nadesapillai, A.P. Urokinase plasminogen activator system: A multifunctional role in tumor progression and metastasis. Clin. Orthop. Relat. Res. 2003, 415, S46–S58. [Google Scholar] [CrossRef]
- Carmeliet, P.; Schoonjans, L.; Kieckens, L.; Ream, B.; Degen, J.; Bronson, R.; de Vos, R.; van den Oord, J.J.; Collen, D.; Mulligan, R.C. Physiological consequences of loss of plasminogen activator gene function in mice. Nature 1994, 368, 419–424. [Google Scholar] [CrossRef]
- Billstrom, A.; Hartley-Asp, B.; Lecander, I.; Batra, S.; Astedt, B. The urokinase inhibitor p-aminobenzamidine inhibits growth of a human prostate tumor in SCID mice. Int. J. Cancer 1995, 61, 542–547. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, H.; Zhou, Z.; Zhong, M.; Chen, Q.; Wang, X.; Zhu, Z. u-PA inhibitor amiloride suppresses peritoneal metastasis in gastric cancer. World J. Surg. Oncol. 2012, 10, 270–276. [Google Scholar] [CrossRef]
- Barber, C.G.; Dickinson, R.P.; Fish, P.V. Selective uPA inhibitors. Part 3: 1-Isoquinolinylguanidines. Bioorg. Med. Chem. Lett. 2004, 14, 3227–3230. [Google Scholar] [CrossRef]
- Kawada, M.; Umezawa, K. Suppression of in vitro invasion of human fibrosarcoma cells by a leupeptin analogue inhibiting the urokinase-plasmin system. Biochem. Biophys. Res. Communs. 1995, 209, 25–30. [Google Scholar] [CrossRef]
- Jedinak, A.; Valachova, M.; Maliar, T.; Sturdik, E. Antiprotease activity of selected Slovak medicinal plants. Pharmazie 2010, 65, 137–140. [Google Scholar]
- Fan, Y.J.; Ohara, A.; Matsuhisa, T. Test for uPA inhibitor of edible plants in Vitro. Zhonghua Yu Fang Yi Xue Za Zhi 2004, 38, 252–256. [Google Scholar]
- Weng, C.J.; Yen, G.C. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: Phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat. Rev. 2012, 38, 76–87. [Google Scholar] [CrossRef]
- Maliar, T.; Drobná, J.; Kraic, J.; Maliarová, M.; Jurovatá, J. Proteinase inhibition and antioxidant activity of selected forage crops. Biologia 2011, 66, 96–103. [Google Scholar] [CrossRef]
- Yang, S.F.; Chu, S.C.; Liu, S.J.; Chen, Y.C.; Chang, Y.Z.; Hsieh, Y.S. Antimetastatic activities of Selaginella tamariscina (Beauv.) on lung cancer cells in vitro and in vivo. J. Ethnopharmacol. 2007, 110, 483–489. [Google Scholar] [CrossRef]
- Betancur-Galvis, L.; Saez, J.; Granados, H.; Salazar, A.; Ossa, J. Antitumor and antiviral activity of Colombian medicinal plant extracts. Mem. Inst. Oswaldo Cruz. 1999, 94, 531–535. [Google Scholar] [CrossRef]
- Robbers, J.E.; Speedie, M.K.; Tyler, V.E. Pharmacognosy and Pharmacobiotechnology, 6th ed.; Williams & Wilkins: Baltimore, MD, USA, 1996; p. 13. [Google Scholar]
- Martinez, T.T.; Martinez, R.R. Medicinal herbs from the Caribbean National Forest (El Yunque), Puerto Rico. Proc. West. Pharmacol. Soc. 2002, 45, 20–22. [Google Scholar]
- Liogier, A.H.; Martorell, L.F. Flora of Puerto Rico and Adjacent Islands: A Systematic Synopsis; Editorial de la Universidad de Puerto Rico : San Juan, PR, USA, 2000. [Google Scholar]
- Cantrell, C.L.; Berhow, M.A.; Phillips, B.S.; Duval, S.M.; Weisleder, D.; Vaughn, S.F. Bioactive crude plant seed extracts from the NCAUR oilseed repository. Phytomedicine 2003, 10, 325–333. [Google Scholar] [CrossRef]
- Gogineni, V.; Nalla, A.; Rao, J. Meningioma: Urokinase plasminogen activator. In Tumors of the Central Nervous System; Hayat, M.A., Ed.; Springer: Heidelberg, Germany, 2012; Volome 7, pp. 47–57. [Google Scholar]
- Chahrour, O.; Cairns, D.; Omran, Z. Small molecule kinase inhibitors as anti-cancer therapeutics. Mini Rev. Med. Chem. 2012, 12, 399–411. [Google Scholar]
- Haverkate, F.; Brakman, P. Fibrin plate assay. In Progress in Chemical Fibrinolysis and Thrombosis; Davidson, J.F., Samama, M.M., Desnoyers, P.C., Eds.; Raven Press: New York, NY, USA, 1975; Volome 1, pp. 151–159. [Google Scholar]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zha, X.; Diaz, R.; Franco, J.J.R.; Sanchez, V.F.; Fasoli, E.; Barletta, G.; Carvajal, A.; Bansal, V. Inhibitors of Urokinase Type Plasminogen Activator and Cytostatic Activity from Crude Plants Extracts. Molecules 2013, 18, 8945-8958. https://doi.org/10.3390/molecules18088945
Zha X, Diaz R, Franco JJR, Sanchez VF, Fasoli E, Barletta G, Carvajal A, Bansal V. Inhibitors of Urokinase Type Plasminogen Activator and Cytostatic Activity from Crude Plants Extracts. Molecules. 2013; 18(8):8945-8958. https://doi.org/10.3390/molecules18088945
Chicago/Turabian StyleZha, Xueqiang, Ricardo Diaz, Jose Javier Rosado Franco, Veronica Forbes Sanchez, Ezio Fasoli, Gabriel Barletta, Augusto Carvajal, and Vibha Bansal. 2013. "Inhibitors of Urokinase Type Plasminogen Activator and Cytostatic Activity from Crude Plants Extracts" Molecules 18, no. 8: 8945-8958. https://doi.org/10.3390/molecules18088945




