Susceptibility of Staphylococcus aureus Clinical Isolates to Propolis Extract Alone or in Combination with Antimicrobial Drugs
Abstract
:1. Introduction
2. Results and Discussion
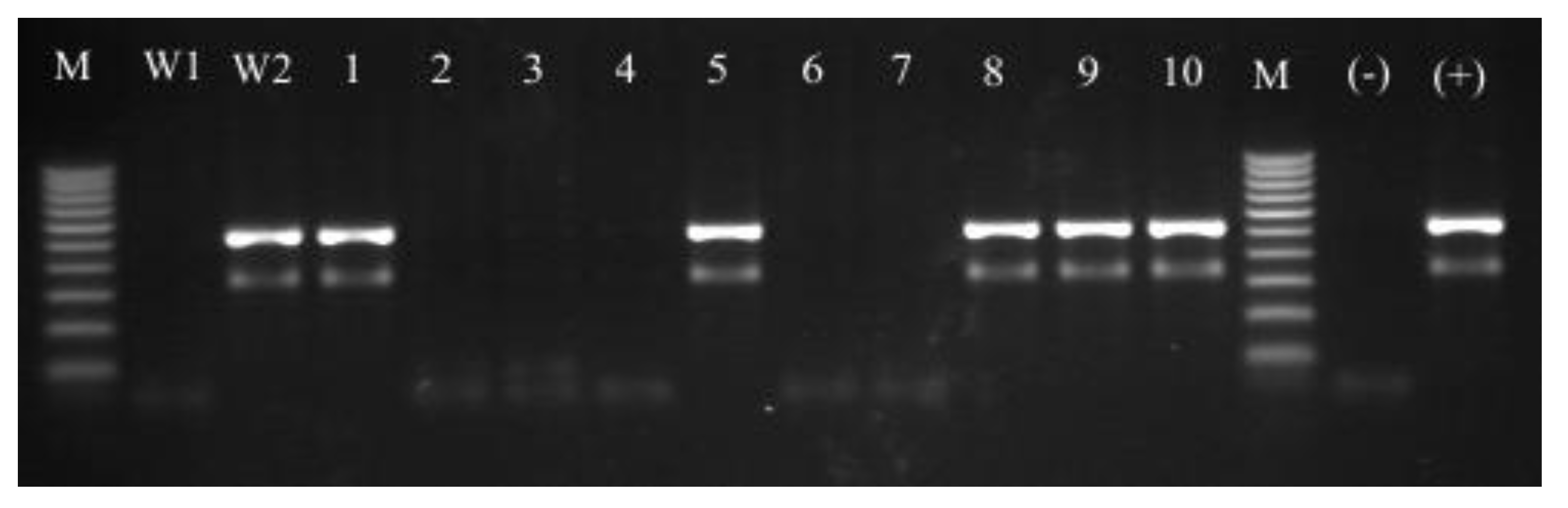
| Strain | Cefoxitin test | mecA | MSSA/MRSA |
|---|---|---|---|
| S. aureus 1 | 17 mm | + | MRSA |
| S. aureus 2 | 34 mm | − | MSSA |
| S. aureus 3 | 34 mm | − | MSSA |
| S. aureus 4 | 33 mm | − | MSSA |
| S. aureus 5 | 22 mm | + | MRSA |
| S. aureus 6 | 34 mm | − | MSSA |
| S. aureus 7 | 34 mm | − | MSSA |
| S. aureus 8 | 13 mm | + | MRSA |
| S. aureus 9 | 16 mm | + | MRSA |
| S. aureus 10 | 14 mm | + | MRSA |
| S. aureus W1 | 35 mm | − | MSSA |
| S. aureus W2 | 21 mm | + | MRSA |
| Strain | 2 | 3 | 4 | 6 | 7 | W1 |
|---|---|---|---|---|---|---|
| MIC EEPP (mg/mL) | 0.39 | 0.39 | 0.78 | 0.78 | 0.78 | 0.39 |
| MBC EEPP (mg/mL) | 1.56 | 1.56 | 3.13 | 3.13 | 1.56 | 3.13 |
| Strain | 1 | 5 | 8 | 9 | 10 | W2 |
|---|---|---|---|---|---|---|
| MIC EEPP (mg/mL) | 0.39 | 0.39 | 0.39 | 0.39 | 0.78 | 0.78 |
| MBC EEPP (mg/mL) | 3.13 | 3.13 | 0.78 | 0.78 | 3.13 | 3.13 |
| Source of variation | df | Sum of squares | Mean squares | Variance explained (%) | F | p |
|---|---|---|---|---|---|---|
| Strain (S) | 11 | 0.67 | 0.06 | 0.71 | 27.2 | <0.001 |
| Time (T) | 2 | 19.8 | 9.9 | 21.10 | 4414.6 | <0.001 |
| Concentration (C) | 11 | 37.65 | 3.42 | 40.12 | 1526.5 | <0.001 |
| S x T | 22 | 1.46 | 0.07 | 1.56 | 29.5 | <0.001 |
| S x C | 121 | 6.74 | 0.06 | 7.18 | 24.8 | <0.001 |
| T x C | 22 | 18.99 | 0.86 | 20.23 | 384.9 | <0.001 |
| SxTxC | 242 | 8.54 | 0.04 | 9.01 | 15.7 | <0.001 |
3. Experimental
3.1. Ethanolic Extract of Polish Propolis (EEPP)
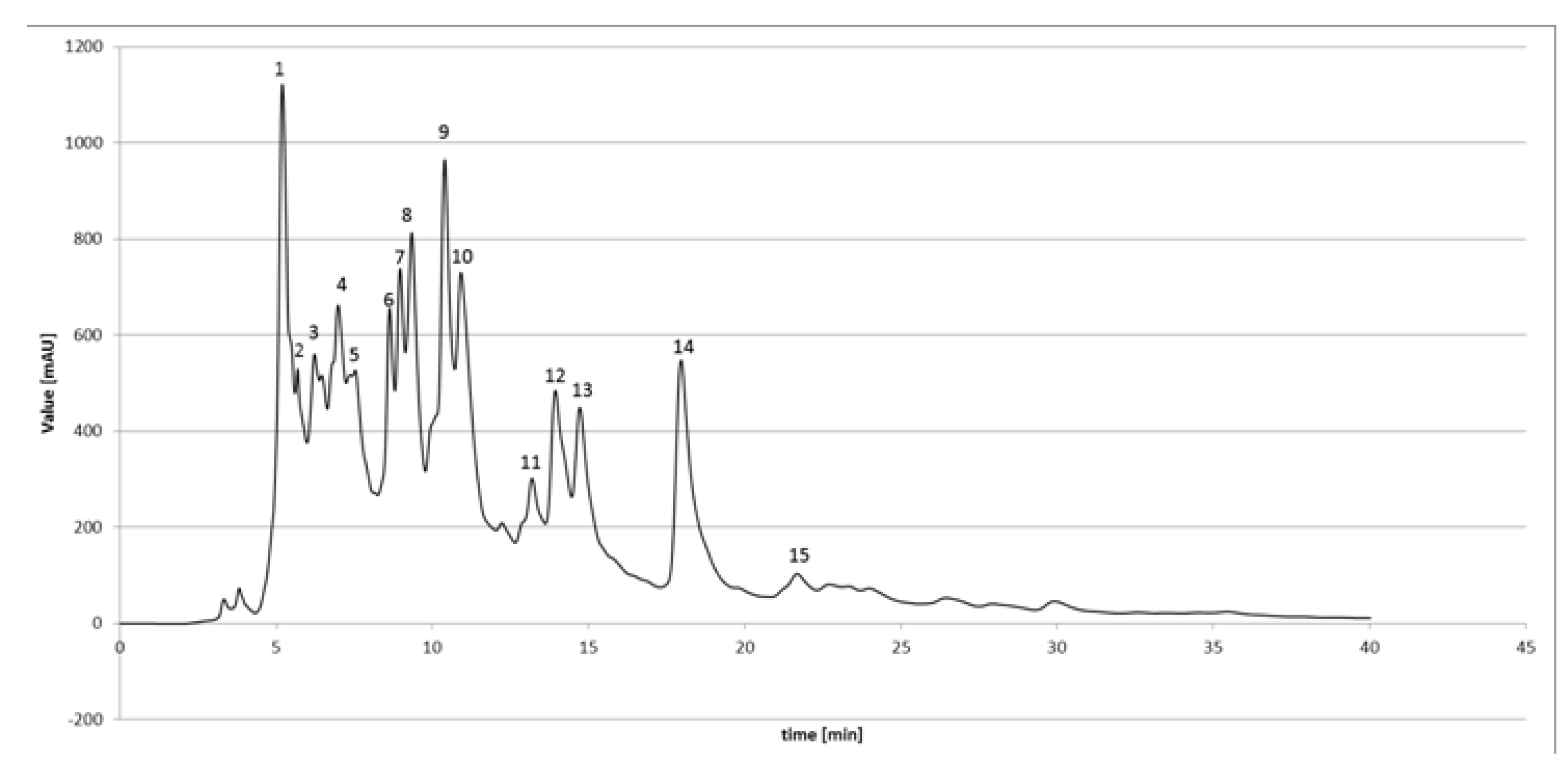
3.2. HPLC-DAD Analysis of EEPP
3.3. Bacterial Strains
3.4. Molecular Identification of Isolated Strains—PCR-RFLP Analysis of dnaJ Gene
3.5. MSSA and MRSA Detection
3.5.1. Cefoxitin Test
3.5.2. The mecA Gene Detection
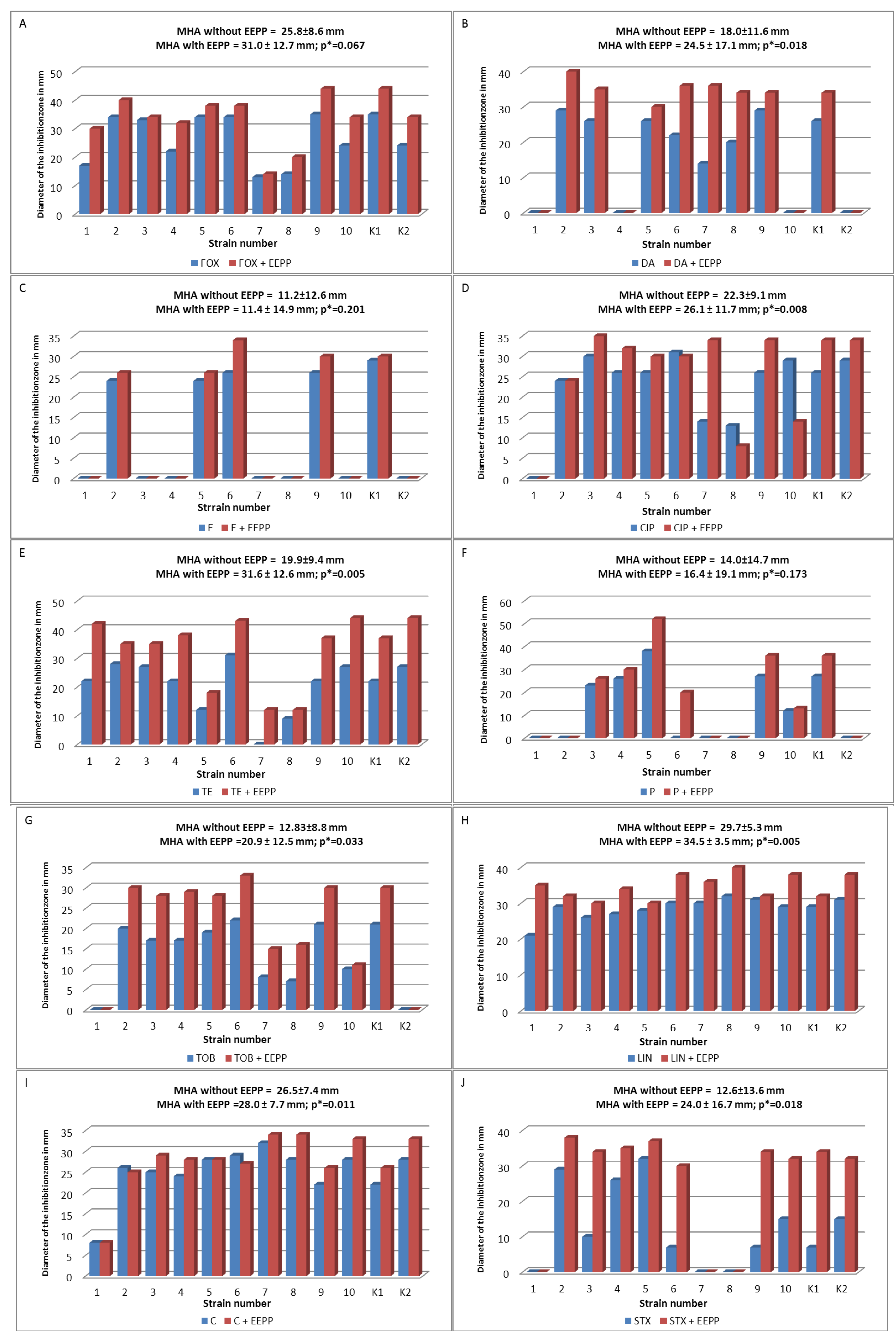
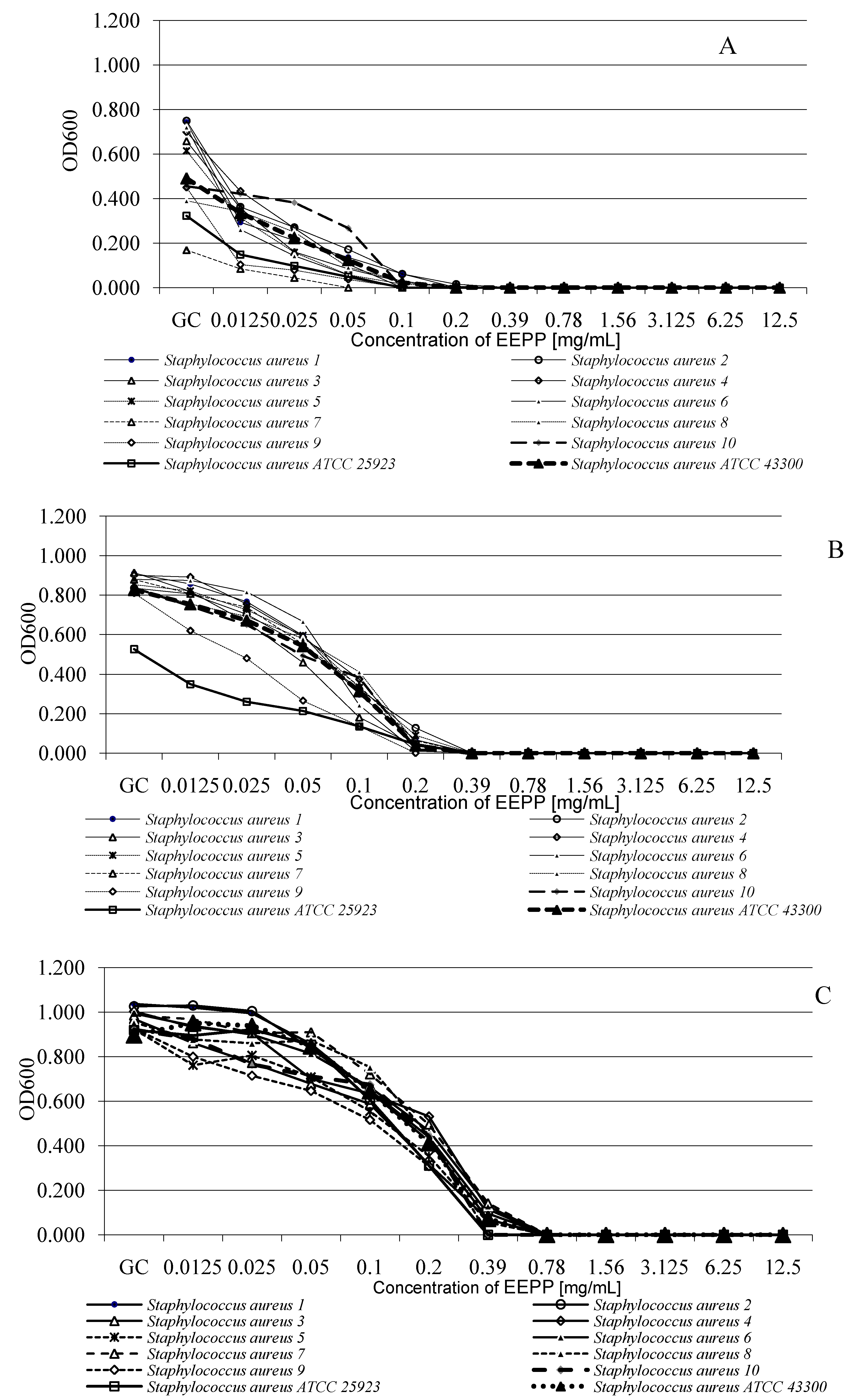
3.6. Antibacterial Susceptibility Testing
3.6.1. Disk Diffusion Method
3.6.2. Microdilution Method
3.7. Statistical Analyses
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Gordon, J.R.; Lowy, F.D. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 2008, 46, 350–359. [Google Scholar] [CrossRef]
- Casey, A.L.; Lambert, P.A.; Elliott, T.S.J. Staphylococci. Int. J. Antimicrobial. Agents 2007, 29, 23–32. [Google Scholar]
- Ippolito, G.; Leone, S.; Lauria, F.N.; Nicastri, E.; Wenzel, R.P. Methicillin-resistant Staphylococcus aureus: The superbug. Int. J. Infect. Dis. 2010, 14, 7–11. [Google Scholar]
- Wertheim, H.F.; Melles, D.C.; Vos, M.C.; van Leeuwen, W.; van Belcum, A.; Verbrugh, H.A.; Nouwen, J.C. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef]
- Liu, G.Y. Molecular Pathogenesis of Staphylococcus aureus Infection. Pediatric Res. 2009, 65, 71–77. [Google Scholar] [CrossRef]
- Bächi, B.B.; Rohrer, S. Factors influencing methicillin resistance in Staphylococci. Arch. Microbiol. 2002, 178, 165–171. [Google Scholar] [CrossRef]
- Cosgrove, S.E.; Qi, Y.; Kaye, K.S.; Harbarth, S.; Karchmer, A.W.; Carmeli, Y. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect. Control. Hosp. Epidemiol. 2005, 26, 166–174. [Google Scholar]
- Bankova, V. Recent trends and important developments in propolis research. Evid. Based Comp. Alter. Med. 2005, 2, 29–32. [Google Scholar] [CrossRef]
- Castaldo, S.; Capasso, F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002, 73, 1–6. [Google Scholar] [CrossRef]
- Paulino, N.; Teixeira, C.; Martins, R.; Scremin, A.; Dirsch, V.M.; Vollmar, A.M.; Abreu, S.R.; de Castro, S.L.; Marcucci, M.C. Evaluation of the analgesic and antiinflammatory effects of a Brazilian green propolis. Planta Med. 2006, 72, 899–906. [Google Scholar] [CrossRef]
- Borrelli, F.; Maffia, P.; Pinto, L.; Ianaro, A.; Russo, A.; Capasso, F.; Ialenti, A. Phytochemical compounds involved in the anti-inflammatory effect of propolis extract. Fitoterapia 2002, 73, 53–63. [Google Scholar] [CrossRef]
- Orsatti, C.L.; Missima, F.; Pagliarone, A.C.; Bachiega, T.F.; Búfalo, M.C.; Araújo Jr, J.P.; Sforcin, J.M. Propolis immunomodulatory action in vivo on Toll-like receptors 2 and 4 expression and on pro-inflammatory cytokines production in mice. Phytother. Res. 2010, 24, 1141–1146. [Google Scholar]
- Orsolić, N.; Knezević, A.H.; Sver, L.; Terzić, S.; Basić, I. Immunomodulatory and antimetastatic action of propolis and related polyphenolic compounds. J. Ethnopharmacol. 2004, 94, 307–315. [Google Scholar] [CrossRef]
- Búfalo, M.C.; Candeias, J.M.; Sforcin, J.M. In vitro cytotoxic effect of Brazilian green propolis on human laryngeal epidermoid carcinoma (HEp-2) cells. Evid. Based Comp. Alter. Med. 2009, 6, 483–487. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Bronikowska, J.; Mertas, A.; Paradysz, A.; Krol, W. Ethanolic extract of propolis (EEP) augments TRAIL-induced apoptotic death in prostate cancer cells. Evid. Based Comp. Alter. Med. 2011. Article ID 535172. [Google Scholar]
- Nakajima, Y.; Shimazawa, M.; Mishima, S.; Hara, H. Water extract of propolis and its main constituents, caffeoylquinic acid derivatives, exert neuroprotective effects via antioxidant actions. Life Sci. 2007, 80, 370–377. [Google Scholar] [CrossRef]
- Teixeira, E.W.; Message, D.; Negri, G.; Salatino, A.; Stringheta, P.C. Seasonal variation, chemical composition and antioxidant activity of Brazilian propolis samples. Evid. Based Comp. Alter. Med. 2010, 7, 307–315. [Google Scholar] [CrossRef]
- Montoro, A.; Almonacid, M.; Serrano, J.; Saiz, M.; Barquinero, J.F.; Barrios, L.; Verdu, G.; Perez, J.; Villaesusa, J.I. Assessment by cytogenetic analysis of the radioprotection properties of propolis extract. Radiat. Prot. Dosimetry 2005, 115, 461–464. [Google Scholar] [CrossRef]
- Kujumgiev, A.; Tsvetkova, I.; Serkedjieva, Y.; Bankova, V.; Chistov, R.; Popov, S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J. Ethnopharmacol. 1999, 64, 235–240. [Google Scholar] [CrossRef]
- Silici, S.; Koç, N.A.; Ayangil, D.; Cankaya, S. Antifungal activities of propolis collected by different races of honeybees against yeasts isolated from patients with superficial mycoses. J. Pharmacol. Sci. 2005, 99, 39–44. [Google Scholar] [CrossRef]
- Kayaoglu, G.; Ömürlü, H.; Akca, G.; Gürel, M.; Gençay, Ö.; Sorkun, K.; Salih, B. Antibacterial activity of Propolis versus conventional endodontic disinfectants against Enterococcus faecalis in infected dentinal tubules. J. Endodont. 2011, 37, 376–381. [Google Scholar] [CrossRef]
- Lu, L.C.; Chen, Y.W.; Chou, C.C. Antibacterial activity of propolis against Staphylococcus aureusInt. J. Food Endodont. 2005, 102, 213–220. [Google Scholar]
- Stojko, J.; Wojtyczka, R.D.; Kabała-Dzik, A.; Stojko, R.; Kępa, M.; Moździerz, A. Comparative studies on the antimicrobial activity of apitherapeutics applied to experimental burn wounds. Pol. J. Environ. Stud. 2007, 16, 612–615. [Google Scholar]
- Silici, S.; Kutluca, S. Chemical composition and antibacterial activity of propolis collected by three different races of honeybees in the same region. J. Ethnopharmacol. 2005, 99, 69–73. [Google Scholar] [CrossRef]
- Wojtyczka, R.D.; Kępa, M.; Idzik, D.; Kubina, R.; Kabała-Dzik, A.; Dziedzic, A.; Wąsik, T.J. In vitro Antimicrobial Activity of Ethanolic Extract of Polish Propolis Against Biofilm Forming Staphylococcus epidermidis Strains. Evid. Based Comp. Alter. Med. 2013, 590703. [Google Scholar]
- Seidel, V.; Peyfoon, E.; Watson, D.G.; Fearnley, J. Comparative study of the antibacterial activity of propolis from different geographical and climatic zones. Phytother. Res. 2008, 22, 1256–1263. [Google Scholar] [CrossRef]
- Trusheva, B.; Popova, M.; Bankova, V.; Simova, S.; Marcucci, M.C.; Miorin, P.L.; da Rocha Pasin, F.; Tsvetkova, I. Bioactive constituents of Brazilian propolis. Evid. Based Comp. Alter. Med. 2006, 3, 249–254. [Google Scholar] [CrossRef]
- Mirzoeva, O.K.; Grishanin, R.N.; Calder, P.C. Antimicrobial action of propolis and some of its components: the effects on growth, membrane potential and motility of bacteria. Microbiol. Res. 1997, 152, 239–246. [Google Scholar] [CrossRef]
- Takaisi–Kikuni, N.B.; Schilcher, H. Electron microscopic and microcalorimetric investigations of the possible mechanism of the antibacterial action of a defined propolis provenance. Planta Med. 1994, 60, 222–227. [Google Scholar] [CrossRef]
- Theuretzbacher, U. Accelerating resistance, inadequate antibacterial drug pipelines and international responses. Int. J. Antimicrob. Agents 2012, 39, 295–299. [Google Scholar] [CrossRef]
- Sha, N.; Huang, H.L.; Zhang, J.Q.; Chen, G.T.; Tao, S.J.; Yang, M.; Li, X.N.; Li, P.; Guo, D.A. Simultaneous quantification of eight major bioactive phenolic compounds in Chinese propolis by high-performance liquid chromatography. Nat. Prod. Commun. 2009, 4, 813–818. [Google Scholar]
- Popova, M.; Chen, C.N.; Chen, P.Y.; Huang, C.Y.; Bankova, V. A validated spectrophotometric method for quantification of prenylated flavanones in pacific propolis from Taiwan. Phytochem. Anal. 2010, 21, 186–191. [Google Scholar]
- Chang, R.; Piló-Veloso, D.; Morais, S.A.; Nascimento, E.A. Analysis of a Brazilian green propolis from Baccharis dracunculifolia by HPLC-APCI-MS and GC-MS. Braz. J. Pharmacognosy 2008, 18, 549–56. [Google Scholar]
- Krol, W.; Scheller, S.; Shani, J.; Pietsz, G.; Czuba, Z. Synergistic effect of ethanolic extract of propolis and antibiotics on the growth of Staphylococcus aureus. Arzneimittelforschung 1993, 43, 607–609. [Google Scholar]
- Orsi, R.O.; Fernandes Junior, A.; Bankova, V.; Sforcin, J.M. Antibacterial effects of Brazilian and Bulgarian propolis and synergistic effects with antibiotics acting on the bacterial DNA and folic acid. Nat. Prod. Res. 2012, 26, 344–349. [Google Scholar] [CrossRef]
- Havsteem, B. Flavonoids, a class of natural products of high pharmacology potency. Biochem. Pharmacol. 1983, 32, 1141–1148. [Google Scholar] [CrossRef]
- Oksuz, H.; Duran, N.; Tamer, C.; Cetin, M.; Silici, S. Effect of propolis in the treatment of experimental Staphylococcus aureus keratitis in rabbits. Ophthalmic Res. 2005, 37, 328–334. [Google Scholar] [CrossRef]
- Fernandes Junior, A.; Balestrin, E.C.; Betoni, J.E.C.; Orsi, R.O.; da Cunha, M.R.S.; Montelli, A.C. Propolis: anti-Staphylococcus aureus activity and synergism with antimicrobial drugs. Mem. Inst. Oswaldo Cruz. 2005, 100, 563–566. [Google Scholar] [CrossRef]
- Nostro, A.; Cellini, L.; di Bartolomeo, S.; Cannatelli, M.A.; di Campli, E.; Procopio, F.; Grande, R.; Marzio, L.; Alonzo, V. Effects of combining extracts (from propolis or Zingiber officinale) with clarithromycin on Helicobacter pylori. Phytother. Res. 2006, 20, 187–190. [Google Scholar] [CrossRef]
- Orsi, R.O.; Sforcin, J.M.; Funari, S.R.C.; Fernandes Junior, A.; Bankova, V. Synergistic effect of propolis and antibiotics on the Salmonella typhi. Braz. J. Microbiol. 2006, 37, 108–112. [Google Scholar]
- Orsi, R.O.; Fernandes, A.; Bankova, V.; Sforcin, J.M. The effects of Brazilian and Bulgarian propolis in vitro against Salmonella Typhi and their synergism with antibiotics acting on the ribosome. Nat. Prod. Res. 2012, 26, 430–437. [Google Scholar] [CrossRef]
- Speciale, A.; Costanzo, R.; Puglisi, S.; Musumeci, R.; Catania, M.R.; Caccamo, F.; Iauk, L. Antibacterial activity of propolis and its active principles alone and in combination with macrolides, beta-lactams and fluoroquinolones against microorganisms responsible for respiratory infections. J. Chemother. 2006, 18, 164–171. [Google Scholar]
- Stepanovic, S.; Antic, N.; Dakic, I.; Svabic-Vlahovic, M. In vitro antimicrobial activity of propolis and synergism between propolis and antimicrobial drugs. Microbiol. Res. 2003, 158, 353–357. [Google Scholar] [CrossRef]
- Boucher, H.W.; Corey, G.R. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2008, 46, 344–349. [Google Scholar] [CrossRef]
- Dziedzic, A.; Kubina, R.; Wojtyczka, R.D.; Kabała-Dzik, A.; Tanasiewicz, M.; Morawiec, T. The Antibacterial Effect of Ethanol Extract of Polish Propolis on Mutans Steptococci and Lactobacilli Isolated from Saliva. Evid. Based Comp. Alter. Med. 2013, 681891. [Google Scholar]
- Kabała-Dzik, A.; Szaflarska-Stojko, E.; Wojtyczka, R.D.; Stojko, A.; Stojko, R.; Pacha, J. Comparative studies on the antimicrobial activity of propolis balm and silver sulphadiazine applied to burn wounds in pigs. Bull. Vet. Inst. Pulawy 2003, 47, 541–545. [Google Scholar]
- Morawiec, T.; Dziedzic, A.; Niedzielska, I.; Mertas, A.; Tanasiewicz, M.; Skaba, D.; Kasperski, J.; Machorowska-Pieniążek, A.; Kucharzewski, M.; Szaniawska, K.; et al. The biological activity of propolis-containing toothpaste on oral health environment in patients who underwent implant-supported prosthodontic rehabilitation. Evid. Based Comp. Alter. Med. 2013, 2013, 704947. [Google Scholar]
- Kilic, A.; Baysallar, M.; Besirbellioglu, B.; Salih, B.; Sorkun, K.; Tanyuksel, M. In vitro antimicrobial activity of propolis against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium. Ann. Microbiol. 2005, 55, 113–117. [Google Scholar]
- Berretta, A.A.; Nascimento, A.P.; Bueno, P.C.; de Oliveira Lima Leite Vaz, M.M.; Marchetti, J.M. Propolis standardized extract (EPP-AF®), an innovative chemically and biologically reproducible pharmaceutical compound for treating wounds. Int. J. Biol. Sci. 2012, 8, 512–521. [Google Scholar]
- Gonsales, G.Z.; Orsi, R.O.; Fernandes Junior, A.; Rodrigues, P.; Funari, S.R.C. Antibacterial activity of propolis collected in different regions of Brazil. J. Venom. Anim. Toxins Incl. Trop. Dis. 2006, 12, 276–284. [Google Scholar]
- Cushine, C.O.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Esimone, C.O.; Iroha, I.R.; Ibezim, E.C.; Okeh, C.O.; Okpana, E.M. In vitro evaluation of the interaction between tea extracts and penicillin G against Staphylococcus aureus. Afr. J. Biotechnol. 2006, 5, 1082–1086. [Google Scholar]
- Shah, M.M.; Iihara, H.; Noda, M.; Song, S.X.; Nhung, P.H.; Ohkusu, K.; Kawamaura, Y.; Ezaki, T. dnaJ gene sequence-based assay for species identification and phylogenetic grouping in the genus Staphylococcus. Int. J. Syst. Evol. Microbiol. 2007, 57, 25–30. [Google Scholar] [CrossRef]
- Hauschild, T.; Stepanovic, S. Identification of Staphylococcus spp. by PCR-restriction fragment length polymorphism analysis of dnaJ gene. J. Clin. Microbiol 2008, 46, 3875–3879. [Google Scholar] [CrossRef]
- Murakami, K.; Minamide, W.; Wada, K.; Nakamura, E.; Teraoka, H.; Watanabe, S. Identification of methicillin-resistant strains of Staphylococci by polymerase chain reaction. J. Clin. Microbiol. 1991, 29, 2240–2244. [Google Scholar]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. EUCAST definitive document E. Def 1.2. Clin. Microbiol. Infect. 2000, 6, 503–508. [CrossRef]
- Mahon, C.R.; Manuselis, J.R.G. Textbook of Diagnostic Microbiology; WB Saunders: Philadelphia, PA, USA, 1995; p. 1134. [Google Scholar]
- Amsterdam, D. Susceptibility Testing of Antimicrobials in Liquid Media. In Antibiotics in Laboratory Medicine, 5th ed.; Loman, V., Ed.; Williams and Wilkins: Philadelphia, PA, USA, 2005; pp. 61–143. [Google Scholar]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. EUCAST discussion document E.dis. 5.1. Clin. Microbiol. Infect. 2003, 9, 1–7.
- Cudic, M.; Condie, B.A.; Weiner, D.J.; Lysenko, E.S.; Xiang, Z.Q.; Insug, O.; Bulet, P.; Otvos, L., Jr. Development of novel antibacterial peptides that kill resistant clinical isolates. Peptides 2002, 23, 2071–2083. [Google Scholar]
- Devienne, K.F.; Raddi, M.S.G. Screening for antimicrobial activity of natural Products using a microplate photometer. Braz. J. Microbiol. 2002, 33, 166–168. [Google Scholar] [CrossRef]
- Schwarz, S.; Silley, P.; Simjee, S.; Woodford, N.; van Duijkeren, E.; Johnson, A.P.; Gaastra, W. Editorial: Assessing the antimicrobial susceptibility of bacteria obtained from animals. J. Antimicrob. Chemother. 2010, 65, 601–604. [Google Scholar] [CrossRef]
- Bär, W.; Bäde-Schumann, U.; Krebs, A.; Cromme, L. Rapid method for detection of minimal bactericidal concentration of antibiotics. J. Microbiol. Methods. 2009, 77, 85–89. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wojtyczka, R.D.; Dziedzic, A.; Idzik, D.; Kępa, M.; Kubina, R.; Kabała-Dzik, A.; Smoleń-Dzirba, J.; Stojko, J.; Sajewicz, M.; Wąsik, T.J. Susceptibility of Staphylococcus aureus Clinical Isolates to Propolis Extract Alone or in Combination with Antimicrobial Drugs. Molecules 2013, 18, 9623-9640. https://doi.org/10.3390/molecules18089623
Wojtyczka RD, Dziedzic A, Idzik D, Kępa M, Kubina R, Kabała-Dzik A, Smoleń-Dzirba J, Stojko J, Sajewicz M, Wąsik TJ. Susceptibility of Staphylococcus aureus Clinical Isolates to Propolis Extract Alone or in Combination with Antimicrobial Drugs. Molecules. 2013; 18(8):9623-9640. https://doi.org/10.3390/molecules18089623
Chicago/Turabian StyleWojtyczka, Robert D., Arkadiusz Dziedzic, Danuta Idzik, Małgorzata Kępa, Robert Kubina, Agata Kabała-Dzik, Joanna Smoleń-Dzirba, Jerzy Stojko, Mieczysław Sajewicz, and Tomasz J. Wąsik. 2013. "Susceptibility of Staphylococcus aureus Clinical Isolates to Propolis Extract Alone or in Combination with Antimicrobial Drugs" Molecules 18, no. 8: 9623-9640. https://doi.org/10.3390/molecules18089623








