Antioxidant and Anti-Fatigue Activities of Phenolic Extract from the Seed Coat of Euryale ferox Salisb. and Identification of Three Phenolic Compounds by LC-ESI-MS/MS
Abstract
:1. Introduction
2. Results and Discussion
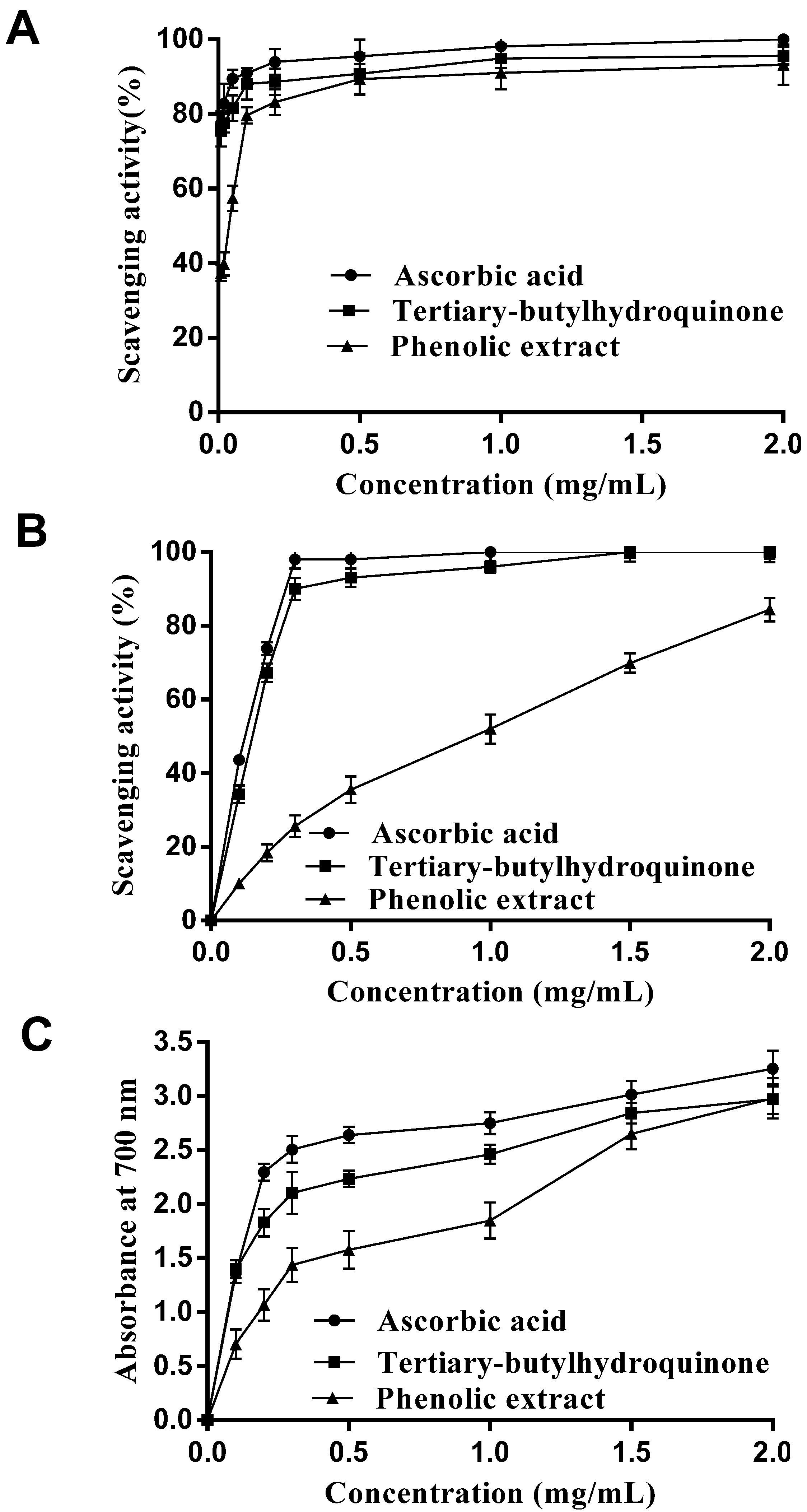
2.1. In Vitro Antioxidant Activity Analysis
2.1.1. DPPH Radical Scavenging Activity
2.1.2. Hydroxyl Radical Scavenging Activity
2.1.3. Reducing Power
2.2. In Vivo Antioxidant Activities Analysis
2.2.1. Effect on the Activities of SOD
2.2.2. Effect on the Activities of CAT
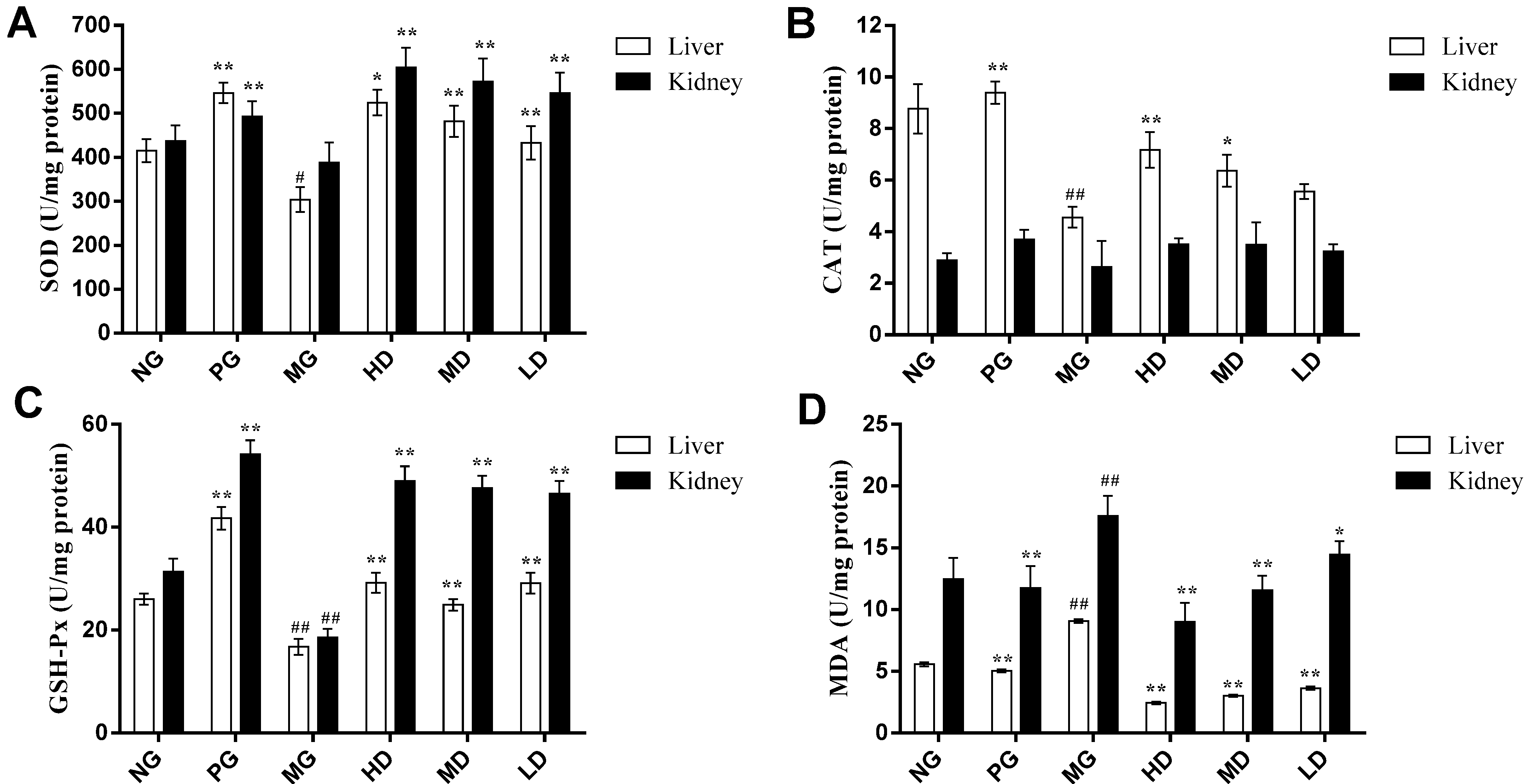
2.2.3. Effect on the Activities of GSH-Px
2.2.4. Effect on the Content of MDA
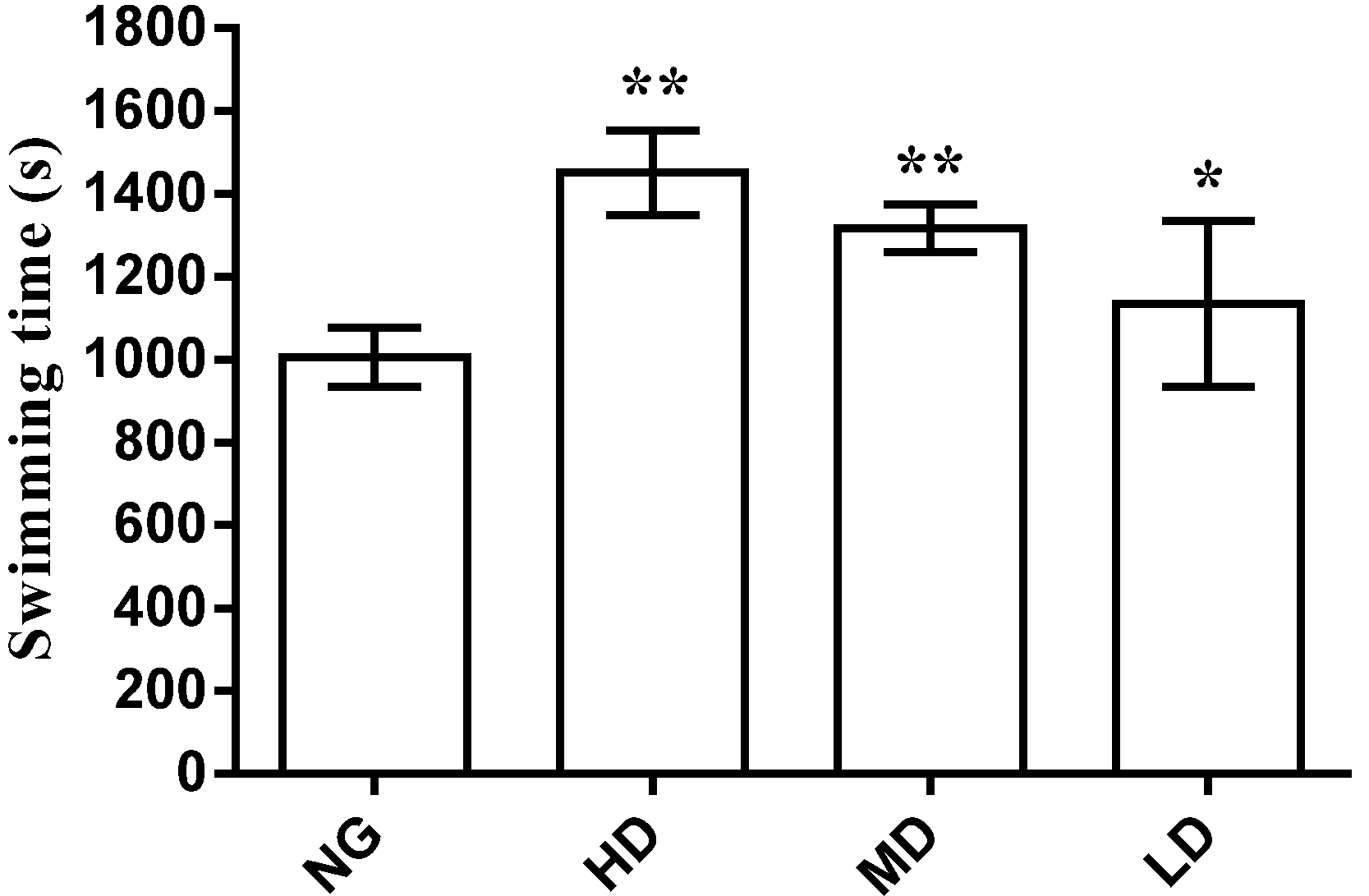
2.3. Anti-Fatigue Effect Analysis
2.3.1. Effect on Swimming Time to Exhaustion
2.3.2. Effect on LDH and BUN Content
| Group | N | Dose (mg/kg) | Serum | Liver | |
|---|---|---|---|---|---|
| LDH (U/L) | BUN (mmol/L) | HG (mg/g) | |||
| NG | 10 | − | 3,583.01 ± 401.78 | 67.02 ± 4.66 | 3.39 ± 1.47 |
| HD | 10 | 400 | 3,397.33 ± 238.35 | 22.98 ± 3.30 ** | 6.61 ± 0.09 ** |
| MD | 10 | 200 | 3,638.64 ± 340.89 | 21.14 ± 5.69 ** | 7.51 ± 2.55 ** |
| LD | 10 | 100 | 3,257.25 ± 407.07 | 25.92 ± 5.79 ** | 2.67 ± 0.07 ** |
2.3.3. Effect on the Content of Hepatic Glycogen
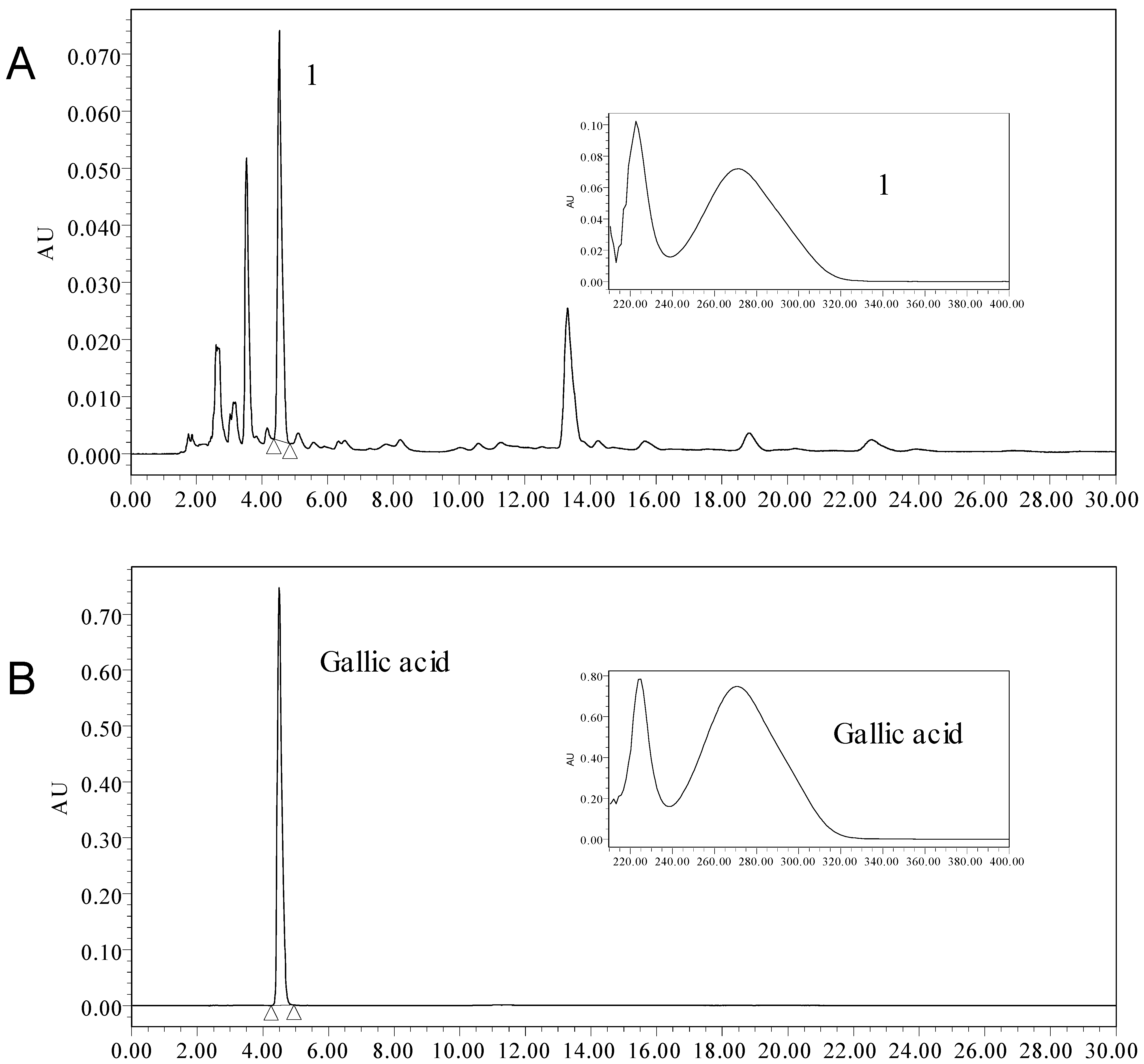
2.4. The Content of Total Phenolic and Gallic Acid
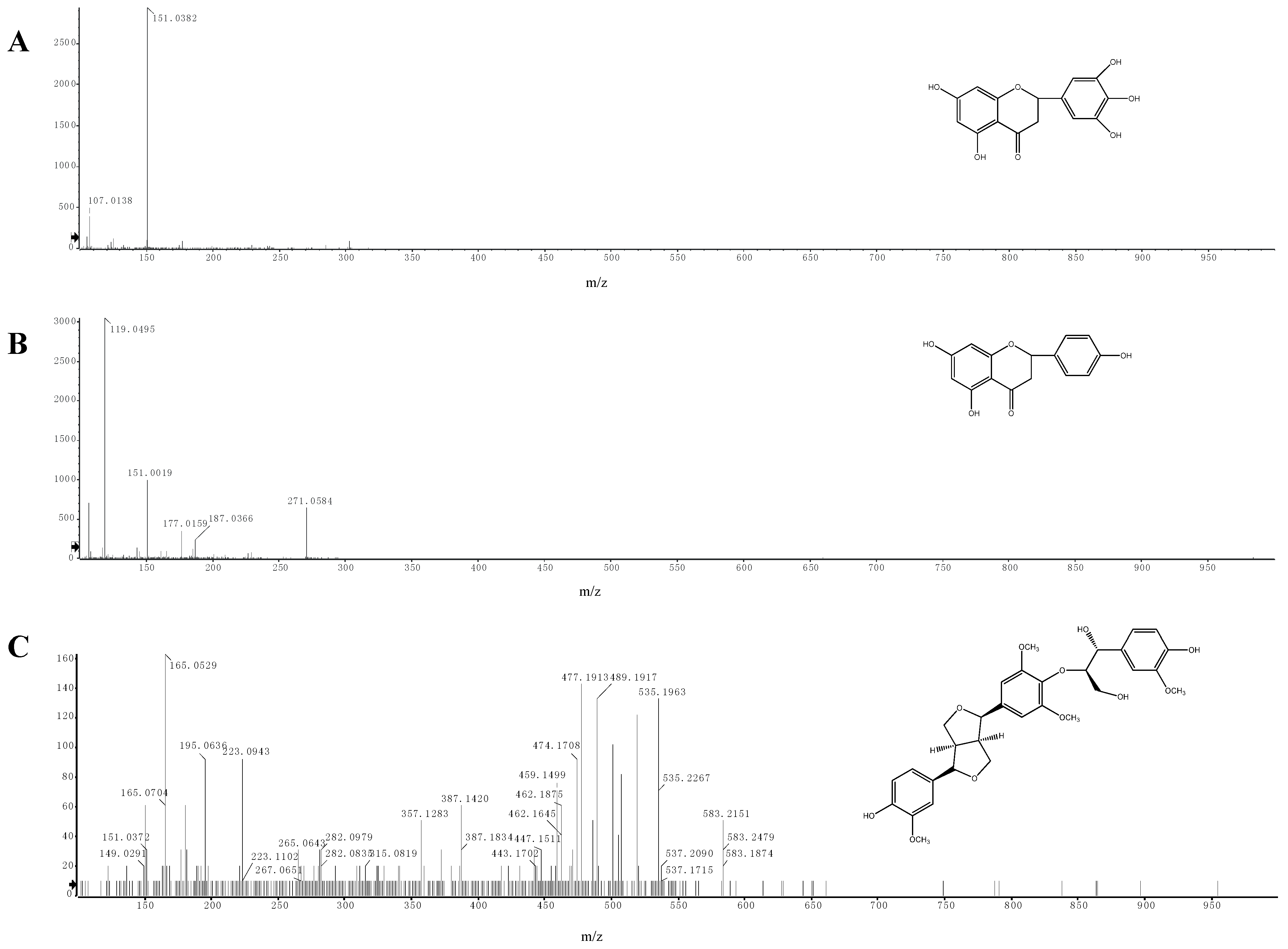
2.5. Identification of Phenolic Compounds by LC-ESI-MS/MS
| Compound Number | Retention time (min) | Molecular formula | [M−H]− m/z | Main fragments m/z | Identification |
|---|---|---|---|---|---|
| 1 | 11.813 | C15H12O7 | 303.0510 | 151.0382 | 5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)chroman-4-one |
| 2 | 14.386 | C15H12O5 | 271.0584 | 151.0019 119.0495 | 5,7,4-trihydroxyflavanone |
| 3 | 14.467 | C31H36O11 | 583.2207 | 387.1420 357.1283 195.0636 165.0529 | buddlenol E |
3. Experimental Section
3.1. Plant Materials
3.2. Chemicals and Reagents
3.3. Animals
3.4. Extraction
3.5. In Vitro Antioxidant Activities
3.5.1. DPPH Radical Scavenging Activity

3.5.2. Hydroxyl Radical Scavenging Activity

3.5.3. Reducing Power
3.6. In Vivo Antioxidant Activities
3.6.1. Experimental Design
3.6.2. Biochemical Assay
3.7. In Vivo Anti-Fatigue Effect
3.7.1. Experimental Design
3.7.2. Exhaustive Swimming Test
3.7.3. Biochemical Assay
3.8. Determination of Total Phenolic Content and Gallic Acid
3.9. Identification of Phenolic Compounds by LC-ESI-MS/MS
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Li, L.J.; Wu, Y.; Cao, B. Research progress of Euryale ferox seeds. China Veget. 2007, 81–83. [Google Scholar]
- Rai, U.N.; Tripathi, R.D.; Vajpayee, P.; Jha, V.; Ali, M.B. Bioaccumulation of toxic metals (Cr, Cd, Pb and Cu) by seeds of Euryale ferox Salisb. (Makhana) W. Chemosphere 2002, 46, 267–272. [Google Scholar] [CrossRef]
- Editorial Committee for Chinese Herbal Medicine of State Administration of Traditional Chinese Medicine, Chinese Herbal Medicine, 1st ed.; Shanghai Science and Technology Press: Shanghai, China, 1999.
- Wang, H. Extraction and determination of total tannin from the Euryale seed coat. Sci. Technol. Food Ind. 2009, 8, 224–226. [Google Scholar]
- Chen, R.; Wu, Q. Optimization of polyphenols extraction technology in Semen Euryales seed coat by response surface methodology. Sci. Technol. Food Ind. 2013, 34, 205–214. [Google Scholar]
- Ningappa, M.B.; Dinesha, R.; Srinivas, L. Antioxidant and free radical scavenging activities of polyphenol-enriched curry leaf (Murraya koenigii L.) extracts. Food Chem. 2008, 106, 720–728. [Google Scholar] [CrossRef]
- Schinella, G.; Mosca, S.; Cienfuegos-Jovellanos, E.; Pasamar, M.Á.; Muguerza, B.; Ramón, D.; Ríos, J.L. Antioxidant properties of polyphenol-rich cocoa products industrially processed. Food Res. Int. 2010, 43, 1614–1623. [Google Scholar] [CrossRef]
- Faller, A.L.K.; Fialho, E. Polyphenol content and antioxidant capacity in organic and conventional plant foods. J. Food Compos. Anal. 2010, 23, 561–568. [Google Scholar] [CrossRef]
- Helmja, K.; Vaher, M.; Pussa, T.; Kaljurand, M. Analysis of the stable free radical scavenging capability of artificial polyphenol mixtures and plant extracts by capillary electrophoresis and liquid chromatography-diode array detection-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 2417–2423. [Google Scholar]
- Sun, J.; Yao, J.; Huang, S.; Long, X.; Wang, J.; García-García, E. Antioxidant activity of polyphenol and anthocyanin extracts from fruits of Kadsura coccinea (Lem.) A.C. Smith. Food Chem. 2009, 117, 276–281. [Google Scholar] [CrossRef]
- Deng, G.F.; Xu, X.R.; Guo, Y.J.; Xia, E.Q.; Li, S.; Wu, S.; Chen, F.; Ling, W.H.; Li, H.B. Determination of antioxidant property and their lipophilic and hydrophilic phenolic contents in cereal grains. J. Funct. Foods 2012, 4, 906–914. [Google Scholar] [CrossRef]
- Liu, J.; M, Y.; Zhao, Z.; Han, Q. Research on antioxidation to delay fatigue. Dairy Ind. 2012, 40, 42–45. [Google Scholar]
- Lonni, A.A.; Longhini, R.; Lopes, G.C.; de Mello, J.C.; Scarminio, I.S. Statistical mixture design selective extraction of compounds with antioxidant activity and total polyphenol content from Trichilia catigua. Anal. Chim. Acta 2012, 719, 57–60. [Google Scholar] [CrossRef]
- Schieber, A.; Stintzing, F.; Carle, R. By-products of plant food processing as a source of functional compounds—Recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Sun, W.K.; Yuan, H.; Xu, W.; Qi, L. Study on anti-oxidative activity of Euryale ferox Salisb. shells extracts. Sci. Technol. Food Ind. 2011, 4, 100–102. [Google Scholar]
- Park, J.H.; Choi, T.S. Polycystic ovary syndrome (PCOS)-like phenotypes in the D-galactose-induced aging mouse model. Biochem. Biophys. Res. Commun. 2012, 427, 701–704. [Google Scholar] [CrossRef]
- Ardestani, A.; Yazdanparast, R. Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem. 2007, 104, 21–29. [Google Scholar] [CrossRef]
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Xiao, J.H.; Xiao, D.M.; Chen, D.X.; Xiao, Y.; Liang, Z.Q.; Zhong, J.J. Polysaccharides from the medicinal mushroom cordyceps taii show antioxidant and immunoenhancing activities in a D-galactose-induced aging mouse model. Evid. Based Compl. Alt. 2012. [Google Scholar] [CrossRef]
- Ben Khaled, H.; Ghlissi, Z.; Chtourou, Y.; Hakim, A.; Ktari, N.; Fatma, M.A.; Barkia, A.; Sahnoun, Z.; Nasri, M. Effect of protein hydrolysates from sardinelle (Sardinella aurita) on the oxidative status and blood lipid profile of cholesterol-fed rats. Food Res. Int. 2012, 45, 60–68. [Google Scholar] [CrossRef]
- Jiang, D.Q.; Guo, Y.; Xu, D.H.; Huang, Y.S.; Yuan, K.; Lv, Z.Q. Antioxidant and anti-fatigue effects of anthocyanins of mulberry juice purification (MJP) and mulberry marc purification (MMP) from different varieties mulberry fruit in China. Food Chem. Toxicol. 2013, 59, 1–7. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, X.; Zhou, G.; Li, C.; Cai, H. Effect of extract from Euryale ferox Seed Shell on Quality of Pork Sausage during Storage. Sci. Technol. Food. Ind. 2013, 34, 48–52. [Google Scholar]
- Noipa, T.; Srijaranai, S.; Tuntulani, T.; Ngeontae, W. New approach for evaluation of the antioxidant capacity based on scavenging DPPH free radical in micelle systems. Food Res. Int. 2011, 44, 798–806. [Google Scholar] [CrossRef]
- Tepe, B.; Degerli, S.; Arslan, S.; Malatyali, E.; Sarikurkcu, C. Determination of chemical profile, antioxidant, DNA damage protection and antiamoebic activities of Teucrium polium and Stachys iberica. Fitoterapia 2011, 82, 237–246. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Zhang, Q.B.; Zhang, Z.S.; Qi, H.M.; Li, P.C. Synthesized oversulphated, acetylated and benzoylated derivatives of fucoidan extracted from Laminaria japonica and their potential antioxidant activity in vitro. Food Chem. 2009, 114, 1285–1290. [Google Scholar] [CrossRef]
- Adesegun, S.A.; Fajana, A.; Orabueze, C.I.; Coker, H.A. Evaluation of antioxidant properties of Phaulopsis fascisepala C.B.Cl. (Acanthaceae). Evid. Based Compl. Alt. 2009, 6, 227–231. [Google Scholar] [CrossRef]
- Prakash, A.; Kumar, A. Pioglitazone alleviates mitochondrial apoptotic pathway and mito-oxidative damage in the D-galactose-induced mouse model. Clin. Exp. Pharmacol. 2013. [Google Scholar] [CrossRef]
- Cui, X.; Zuo, P.; Zhang, Q.; Li, X.; Hu, Y.; Long, J.; Packer, L.; Liu, J. Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: Protective effects of R-alpha-lipoic acid. J. Neurosci. Res. 2006, 84, 647–654. [Google Scholar] [CrossRef]
- Kowald, A.; Hamann, A.; Zintel, S.; Ullrich, S.; Klipp, E.; Osiewacz, H.D. A systems biological analysis links ROS metabolism to mitochondrial protein quality control. Mech. Ageing Dev. 2012, 133, 331–337. [Google Scholar] [CrossRef]
- Chen, H.; Yu, M.; Li, M.; Zhao, R.; Zhu, Q.; Zhou, W.; Lu, M.; Lu, Y.; Zheng, T.; Jiang, J. Polymorphic variations in manganese superoxide dismutase (MnSOD), glutathione peroxidase-1 (GPX1), and catalase (CAT) contribute to elevated plasma triglyceride levels in Chinese patients with type 2 diabetes or diabetic cardiovascular disease. Mol. Cell. Biochem. 2012, 1–7. [Google Scholar]
- He, J.; Huang, B.; Ban, X.; Tian, J.; Zhu, L.; Wang, Y. In vitro and in vivo antioxidant activity of the ethanolic extract from Meconopsis quintuplinervia. J. Ethnopharmacol. 2012, 141, 104–110. [Google Scholar] [CrossRef]
- You, L.; Ren, J.; Yang, B.; Regenstein, J.; Zhao, M. Antifatigue activities of loach protein hydrolysates with different antioxidant activities. J. Agric. Food Chem. 2012, 60, 12324–12331. [Google Scholar] [CrossRef]
- Choi, E.H.; Kang, J.I.; Cho, J.Y.; Lee, S.H.; Kim, T.S.; Yeo, I.H.; Chun, H.S. Supplementation of standardized lipid-soluble extract from maca (Lepidium meyenii) increases swimming endurance capacity in rats. J. Funct. Foods 2012, 4, 568–573. [Google Scholar] [CrossRef]
- Tan, W.; Yu, K.Q.; Liu, Y.Y.; Ouyang, M.Z.; Yan, M.H.; Luo, R.; Zhao, X.S. Anti-fatigue activity of polysaccharides extract from Radix Rehmanniae Preparata. Int. J. Biol. Macromol. 2012, 50, 59–62. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. In vitro antioxidant activity and in vivo anti-fatigue effect of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. Food Chem. 2011, 124, 188–194. [Google Scholar] [CrossRef]
- Zhao, L.; Yan, W.; Xiang, H.; Wang, X.; Qiao, H. Proteomic investigation of changes in rat skeletal muscle after exercise-induced fatigue. Biol. Res. 2012, 45, 75–80. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, Y.; Liu, P.; Zhao, H.X.; Zhou, X.J.; Wei, Y.M. Effects of a chinese traditional formula Kai Xin San (KXS) on chronic fatigue syndrome mice induced by forced wheel running. J. Ethnopharmacol. 2012, 139, 19–25. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Habtemariam, S.; Jafari, M.; Sureda, A.; Nabavi, S.M. Protective role of gallic acid on sodium fluoride induced oxidative stress in rat brain. Bull. Environ. Contam. Toxicol. 2012, 89, 73–77. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Nabavi, S.M.; Habtemariam, S.; Moghaddam, A.H.; Sureda, A.; Jafari, M.; Latifi, A.M. Hepatoprotective effect of gallic acid isolated from Peltiphyllum peltatum against sodium fluoride-induced oxidative stress. Ind. Crop. Prod. 2013, 44, 50–55. [Google Scholar] [CrossRef]
- Lu, Z.; Nie, G.; Belton, P.S.; Tang, H.; Zhao, B. Structure-activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem. Int. 2006, 48, 263–274. [Google Scholar] [CrossRef]
- Abdelwahed, A.; Bouhlel, I.; Skandrani, I.; Valenti, K.; Kadri, M.; Guiraud, P.; Steiman, R.; Mariotte, A.M.; Ghedira, K.; Laporte, F.; et al. Study of antimutagenic and antioxidant activities of gallic acid and 1,2,3,4,6-pentagalloylglucose from Pistacia lentiscus. Confirmation by microarray expression profiling. Chem. Biol. Interact. 2007, 165, 1–13. [Google Scholar] [CrossRef]
- Hanhineva, K.; Rogachev, I.; Aura, A.M.; Aharoni, A.; Poutanen, K.; Mykkänen, H. Identification of novel lignans in the whole grain rye bran by non-targeted LC-MS metabolite profiling. Metabolomics 2012, 8, 399–409. [Google Scholar] [CrossRef]
- Li, M.H.; Yang, X.Q.; Wan, Z.J.; Yang, Y.B.; Li, F.; Ding, Z.T. Chemical constituents of the seeds of Euryale ferox. Chin. J. Nat. Med. 2007, 5, 24–26. [Google Scholar]
- Song, C.W.; Wang, S.M.; Zhou, L.L.; Hou, F.F.; Wang, K.J.; Han, Q.B.; Li, N.; Cheng, Y.X. Isolation and identification of compounds responsible for antioxidant capacity of Euryale ferox seeds. J. Agric. Food Chem. 2011, 59, 1199–1204. [Google Scholar] [CrossRef]
- Zhang, G.W.; He, L.; Hu, M.M. Optimized ultrasonic-assisted extraction of flavonoids from Prunella vulgaris L. and evaluation of antioxidant activities in vitro. Innov. Food Sci. Emerg. 2011, 12, 18–25. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Q.; Wu, Y.; Chen, G.; Yue, W.; Liang, Q. Response surface optimized ultrasonic-assisted extraction of flavonoids from Sparganii rhizoma and evaluation of their in vitro antioxidant activities. Molecules 2012, 17, 6769–6783. [Google Scholar] [CrossRef]
- Jin, S.L.; Yin, Y.G. In vivo antioxidant activity of total flavonoids from indocalamus leaves in aging mice caused by D-galactose. Food Chem. Toxicol. 2012, 50, 3814–3818. [Google Scholar] [CrossRef]
- Huang, L.Z.; Huang, B.K.; Ye, Q.; Qin, L.P. Bioactivity-guided fractionation for anti-fatigue property of Acanthopanax senticosus. J. Ethnopharmacol. 2011, 133, 213–219. [Google Scholar] [CrossRef]
- Prasad, K.N.; Hassan, F.A.; Yang, B.; Kong, K.W.; Ramanan, R.N.; Azlan, A.; Ismail, A. Response surface optimisation for the extraction of phenolic compounds and antioxidant capacities of underutilised Mangifera pajang Kosterm. peels. Food Chem. 2011, 128, 1121–1127. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Xu, X.L.; Zhou, G.H.; Li, C.B.; Li, L. Antioxodative stability of extracts from Euryale ferox seed shell. Sci. Technol. Food Ind. 2012, 33, 57–61. [Google Scholar]
- Sample Availability: Samples of the extract from the seed coatof Euryale ferox Salisb. are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, C.; Chen, R.; Wang, X.S.; Shen, B.; Yue, W.; Wu, Q. Antioxidant and Anti-Fatigue Activities of Phenolic Extract from the Seed Coat of Euryale ferox Salisb. and Identification of Three Phenolic Compounds by LC-ESI-MS/MS. Molecules 2013, 18, 11003-11021. https://doi.org/10.3390/molecules180911003
Wu C, Chen R, Wang XS, Shen B, Yue W, Wu Q. Antioxidant and Anti-Fatigue Activities of Phenolic Extract from the Seed Coat of Euryale ferox Salisb. and Identification of Three Phenolic Compounds by LC-ESI-MS/MS. Molecules. 2013; 18(9):11003-11021. https://doi.org/10.3390/molecules180911003
Chicago/Turabian StyleWu, ChengYing, Rong Chen, Xin Sheng Wang, Bei Shen, Wei Yue, and Qinan Wu. 2013. "Antioxidant and Anti-Fatigue Activities of Phenolic Extract from the Seed Coat of Euryale ferox Salisb. and Identification of Three Phenolic Compounds by LC-ESI-MS/MS" Molecules 18, no. 9: 11003-11021. https://doi.org/10.3390/molecules180911003




