Influence of Temperature on the Enantioselectivity of Koga Tetraamines on Amylose Chiral Stationary Phases
Abstract
:1. Introduction
2. Results and Discussion
| Temperature (°C) | Koga bases | |||||
|---|---|---|---|---|---|---|
| k' | α | |||||
| RR | meso | SS | RR/SS | meso/SS | RR/meso | |
| 10 | 0.88 | 0.58 | 0.32 | 2.76 | 1.83 | 1.51 |
| 15 | 0.77 | 0.52 | 0.30 | 2.59 | 1.74 | 1.49 |
| 20 | 0.66 | 0.46 | 0.27 | 2.43 | 1.68 | 1.47 |
| 25 | 0.61 | 0.41 | 0.26 | 2.37 | 1.62 | 1.45 |
| 30 | 0.53 | 0.37 | 0.24 | 2.17 | 1.52 | 1.42 |
| 35 | 0.46 | 0.33 | 0.23 | 2.06 | 1.47 | 1.40 |
| 40 | 0.41 | 0.30 | 0.21 | 1.96 | 1.43 | 1.37 |
| Temperature (°C) | Koga bases | |||||
|---|---|---|---|---|---|---|
| k' | α | |||||
| RR | meso | SS | RR/SS | meso/RR | meso/SS | |
| 10 | 0.49 | 0.83 | 0.43 | 1.14 | 1.71 | 1.95 |
| 15 | 0.47 | 0.80 | 0.41 | 1.14 | 1.71 | 1.96 |
| 20 | 0.46 | 0.78 | 0.40 | 1.15 | 1.70 | 1.95 |
| 25 | 0.42 | 0.69 | 0.37 | 1.15 | 1.64 | 1.88 |
| 30 | 0.38 | 0.61 | 0.34 | 1.14 | 1.59 | 1.81 |
| 35 | 0.36 | 0.56 | 0.32 | 1.14 | 1.55 | 1.76 |
| 40 | 0.33 | 0.49 | 0.29 | 1.12 | 1.52 | 1.70 |
| Eluent | Plot Region ** | Koga Base | ΔH (k cal/mol) | Pair of optical Isomer | ΔΔH (k cal/mol) | ΔΔS (kcal/mol. K) |
|---|---|---|---|---|---|---|
| 50%IPA–50%MeOH | R, R | −18.230 | RR/SS | −8.1 | −0.005 | |
| meso | −16.235 | RR/meso | −2.3 | −0.021 | ||
| S, S | −10.163 | meso/SS | −5.9 | −0.016 | ||
| 85%IPA–15%MeOH | I | meso | −17.062 | meso/SS | −5.0 | −0.003 |
| R, R | −12.824 | RR/SS | −1.1 | −0.009 | ||
| S, S | −12.042 | meso/RR | −3.9 | −0.001 | ||
| II | meso | −4.511 | meso/SS | −0.1 | −0.006 | |
| R, R | −3.879 | RR/SS | −1.4 | 0.001 | ||
| S, S | −4.388 | meso/RR | −1.5 | −0.005 |
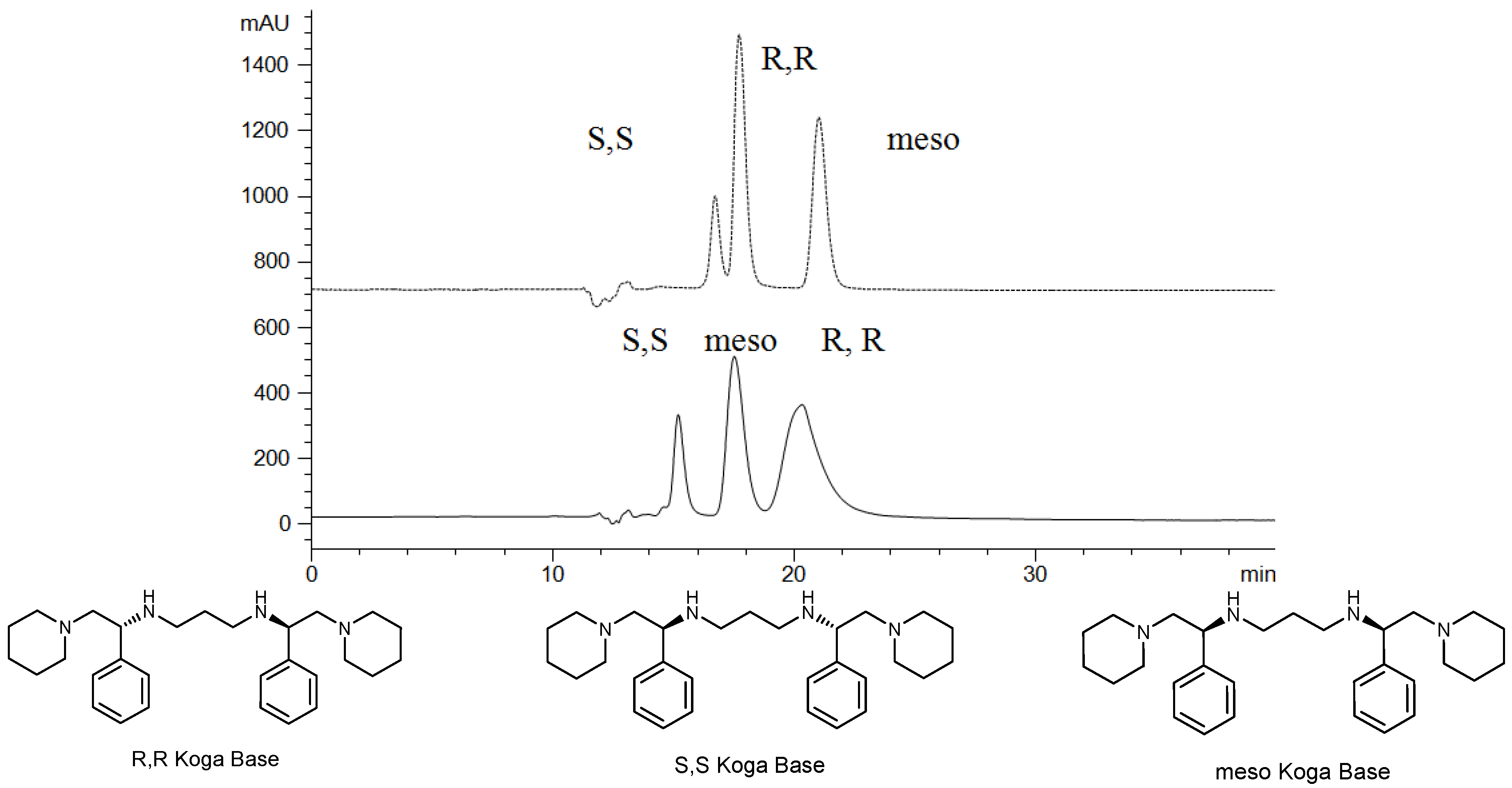
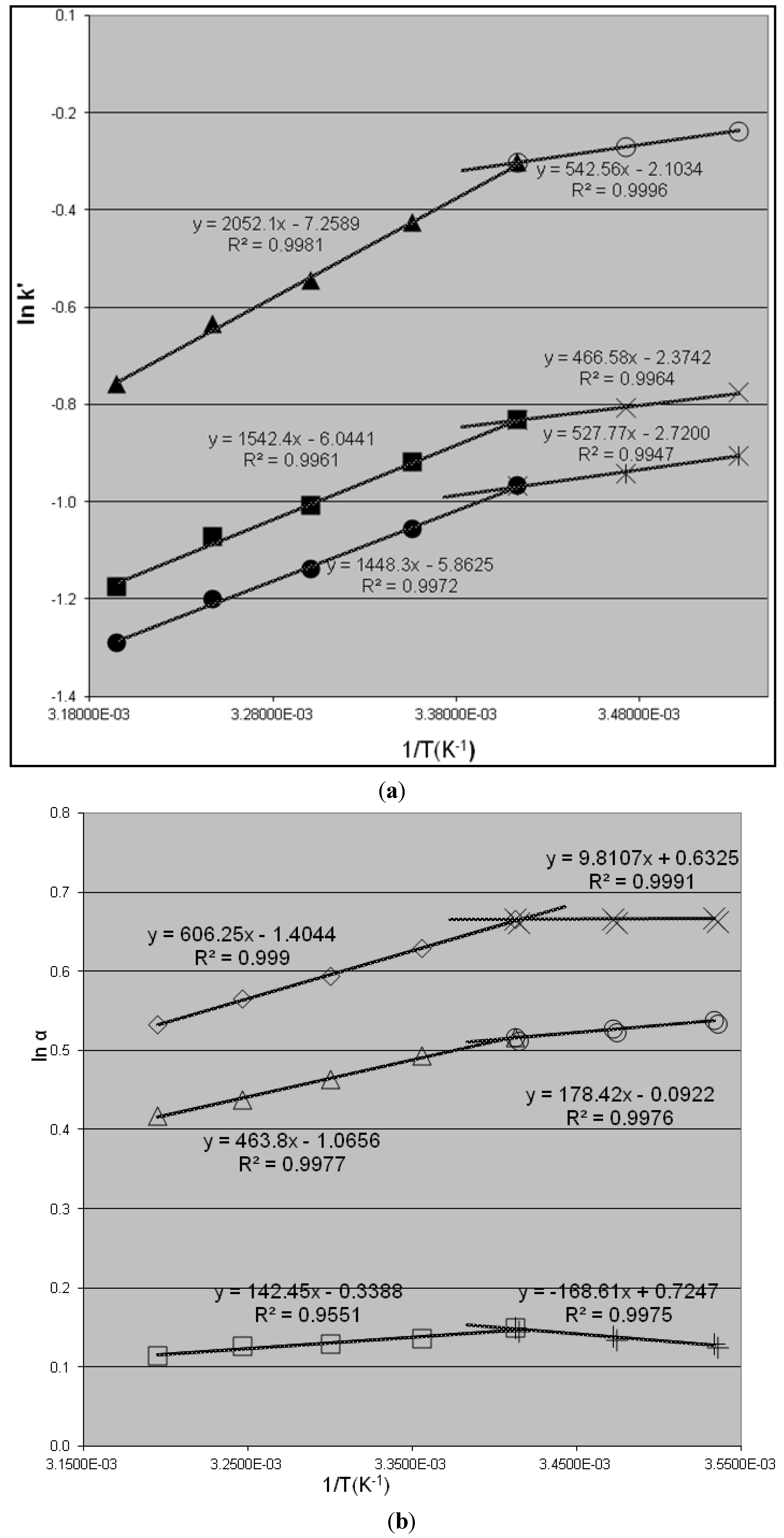
2.1. Linear van’t Hoff Plot

2.2. Non-Linear van’t Hoff Curves
3. Experimental
3.1. Equipment
3.2. Reagents
3.3. Sample Preparation
3.4. Mobile Phase Preparation
3.5. Chromatography
4. Conclusions
Conflicts of Interest
References
- Gebreyohannes, K.G.; McGuffin, V.L. Thermodynamic and kinetic study of chiral separations of coumarin-based anticoagulants on derivatized amylose stationary phase. J. Chromatogr. A 2010, 1217, 5901–5912. [Google Scholar] [CrossRef]
- Lämmerhofer, M. Chiral recognition by enantioselective liquid chromatography: Mechanisms and modern chiral stationary phases. J. Chromatogr. A 2010, 1217, 814–856. [Google Scholar] [CrossRef]
- Frizzle, M.J.; Caille, S.; Marshall, T.L.; McRae, K.; Nadeau, K.; Guo, G.; Wu, S.; Martinelli, M.J.; Moniz, G.A. Dynamic biphasic counter ion exchange in a configurationally stable Aziridinium ion: Efficient synthesis and isolation of a koga C2-symmetric tetraamine base. Org. Proc. R. D. 2007, 11, 215–222. [Google Scholar] [CrossRef]
- Guo, H.-X.; Wu, S.; Nadeau, K.; Moniz, G.A.; Caille, S. Effects of solvent on chiral and enantiomeric separation of Koga bases using derivatized amylose chiral stationary phase. Chirality 2009, 22, 50–55. [Google Scholar]
- Wenslow, R.W.; Wang, T. Solid-state NMR characterization of amylose tris-(3, 5-dimethylphenylcarbamate) chiral stationary-phase structure as a function of mobile-phase composition. Anal. Chem. 2001, 73, 4190–4195. [Google Scholar] [CrossRef]
- Wang, F.; Wenslow, R.W.; Dowling, T.M.; Mueller, K.T.; Santor, I.; Wyvratt, J.M. Characterization of a thermally induced irreversible conformational transition of amylose tris-(3, 5-dimethylphenylcarbamate) chiral stationary phase in enantioseparation of dihydropyrimidinone acid by quasi-equilibrated liquid chromatography and solid-state NMR. Anal. Chem. 2003, 75, 5877–5885. [Google Scholar] [CrossRef]
- Kasat, R.B.; Zvinevich, Y.; Hillhouse, H.W.; Thomson, K.T.; Wang, N.H.L.; Franses, E.I. Direct probing of sorbent−solvent interactions for amylose tris(3,5-dimethylphenylcarbamate) using infrared spectroscopy, X-ray diffraction, solid-state NMR, and dft modeling. J. Phys. Chem. 2006, B110, 14114–14122. [Google Scholar]
- Watabe, K.; Charles, R.; Gil-Av, E. Temperature dependent inversion of elution sequence in the resolution of α-amino acid enantiomers on chiral diamide selectors. Angew. Chem. Int. Ed. 1989, 28, 192–194. [Google Scholar] [CrossRef]
- Koppenhoefer, B.; Lin, B. Thermodynamic properties of enantiomers of underivatized diols versus the cyclic carbonates in gas chromatography on Chirasil-Val. J. Chromatogr. A 1989, 481, 17–26. [Google Scholar] [CrossRef]
- Schurig, V.; Ossig, J.; Ink, R. Evidence for temperature dependent reversal of the enantiomselectivity in complexation gas chromatography on chiral phases. Angew. Chem. Int. Ed. 1989, 28, 194–196. [Google Scholar] [CrossRef]
- Levkin, P.; Maier, N.; Linder, W.; Schurig, V. A practical method for the quantitative assessment of non-enantioselective versus enantioselective interactions encountered in liquid chromatography on brush-type chiral stationary phase. J. Chromatogr. A 2012, 1269, 270–278. [Google Scholar]
- Cuthbertson, E.; O’Brien, P.; Towers, T. Practical one-step synthesis of koga’s chiral bases. Synthesis 2001, 5, 693–695. [Google Scholar]
- Ichida, A.; Shibata, T. Cellulose Derivatives as Chiral Stationary Phases. In Chromatographic Chiral Separations; Zief, M., Crane, L., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1988. [Google Scholar]
- Beesley, T.B.; Scott, R.P.W. Chiral Chromatography; John Willey & Sons: New York, NY, USA, 1998. [Google Scholar]
- Gilpin, R.K.; Sisco, W.R. Effect of temperature on precision of retention measurements in liquid chromatography. J. Chromatogr. A 1980, 194, 285–295. [Google Scholar]
- Pirkle, W.H.; Schreiner, J.L. Chiral HPLC (high-performance liquid chromatographic) stationary phases. 4. Separation of the enantiomers of bi-, beta-naphthols and analogs. J. Org. Chem. 1981, 46, 4988–4991. [Google Scholar]
- Akarya, J.N.; Taylor, D.R. The role of the mobile phase in the resolution of racemic esters of aminoacids by normal phase high-performance liquid chromatography. Chromatographia 1988, 25, 636–638. [Google Scholar]
- Yonker, C.R.; Smith, R.D. Practical supercritical fluid chromatography and extraction. J. Chromatogr. 1986, 351, 211–218. [Google Scholar]
- Kazusaki, M.; Kawabatta, H.; Matsukura, H. Influence of temperature on enantioseparation employing an amylose-derivative stationary phase. J. Liq. Chromatogr. Rel. Technol. 2000, 23, 2937–2946. [Google Scholar]
- O’Brien, T.; Crocker, L.; Thompson, R.; Thompson, K.; Toma, P.H.; Conlon, D.A.; Feibush, B.; Moeder, C.; Bicker, G.; Grinberg, N. Mechanistic aspects of chiral discrimination on modified cellulose. Anal. Chem. 1997, 69, 1999–2007. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Guo, H.-X.; Wu, S.; Sun, J. Influence of Temperature on the Enantioselectivity of Koga Tetraamines on Amylose Chiral Stationary Phases. Molecules 2014, 19, 9-21. https://doi.org/10.3390/molecules19010009
Guo H-X, Wu S, Sun J. Influence of Temperature on the Enantioselectivity of Koga Tetraamines on Amylose Chiral Stationary Phases. Molecules. 2014; 19(1):9-21. https://doi.org/10.3390/molecules19010009
Chicago/Turabian StyleGuo, Hong-Xun, Steven Wu, and Jingshun Sun. 2014. "Influence of Temperature on the Enantioselectivity of Koga Tetraamines on Amylose Chiral Stationary Phases" Molecules 19, no. 1: 9-21. https://doi.org/10.3390/molecules19010009




