Efficient Biotransformation of Polysialogangliosides for Preparation of GM1 by Cellulosimicrobium sp. 21
Abstract
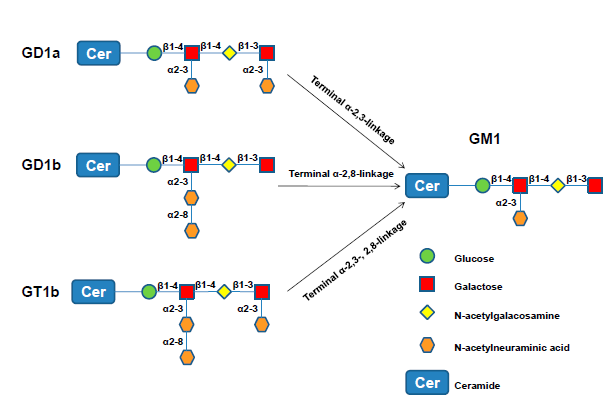
:1. Introduction
2. Results and Discussion
2.1. Screening of Microorganisms for Biotransformation
| Strain | Content of GM1 (w/w, %) | Conversion Time (h) |
|---|---|---|
| No. 2 | 45.5 | 72 |
| No. 4 | 37.0 | 72 |
| No. 5 | 46.1 | 48 |
| No.21 | 46.2 | 20 |

2.2. Optimization of Conditions for Biotransformation
2.2.1. Effect of Carbon Sources on Biotransformation

2.2.2. The Optimal Time for Adding Substrate and Concentration of Substrate


2.2.3. The Optimal Biotransformation Temperature and pH

2.3. Scale-Up of Conversion from Polysialogangliosides to GM1 in 5 L Bioreactor


2.4. Analysis of GM1


| Residue | Carbon | Chemical Shift (ppm) |
|---|---|---|
| β-d-Glc | C-1 | 102.14 |
| C-4 | 78.49 | |
| β-d-Gal | C-1 | 102.17 |
| C-3 | 73.91 | |
| C-4 | 77.19 | |
| α-d-NeuAc | C-1 | 174.51 |
| C-2 | 101.49 | |
| N–C=O | 174.23 | |
| β-d-GalNAc | C-1 | 101.99 |
| C-3 | 80.09 | |
| N–C=O | 173.78 | |
| β-d-Gal | C-1 | 104.36 |
| Ceramide | C-1-3 | 51.31–68.16 |
| C-4 | 134.06 | |
| C-5 | 128.89 | |
| C-6-17 | 21.83–30.40 | |
| C-18 | 13.60 | |
| Fatty acid chains | N–C=O | 173.78 |
| C-2-15 | 21.83–30.40 | |
| C-16 | 13.60 |
3. Experimental Section
3.1. Materials
3.2. Analytical Methods
3.3. Strain Screening
3.4. 16S rDNA Gene Sequencing
3.5. Enzymatic Activity Assay of Sialidase
3.6. Cultivation and Biotransformation
3.7. Preparative Scale Biotransformation
3.8. Separation and Purification of GM1
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McCluer, R.H. Chemistry of gangliosides. Chem. Phys. Lipids 1970, 5, 220–234. [Google Scholar] [CrossRef]
- Nekrasov, E.; Hubl, U. Gangliosides. In Sialobiology: Structure, Biosynthesis and Fuction; Tiralongo, J., Martinez-Duncker, I., Eds.; Bentham Science Publishers: Sharjah, UAE, 2013; Chapter 10; pp. 313–380. [Google Scholar]
- Nobile-Orazio, E.; Carpo, M.; Scarlato, G. Gangliosides: Their role in clinical neurology. Drugs 1994, 47, 576–585. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Svennerholm, L.; Fredman, P. A procedure for the quantitative isolation of brain gangliosides. Biochim. Biophys. Acta 1980, 617, 97–109. [Google Scholar] [CrossRef]
- Tettamanti, G.; Bonali, F.; Marchesini, S.; Zambotti, V. A new procedure for the extraction, purification and fractionation of brain gangliosides. Biochim. Biophys. Acta 1973, 296, 160–170. [Google Scholar] [CrossRef]
- Colarow, L.; Turini, M.; Teneberg, S.; Berger, A. Characterization and biological activity of gangliosides in buffalo milk. Biochim. Biophys. Acta 2003, 1631, 94–106. [Google Scholar] [CrossRef]
- Mauri, L.; Casellato, R.; Kirschner, G.; Sonnino, S. A procedure of the preparation of GM3 ganglioside from GM1-lactone. Glycoconj. J. 1999, 16, 197–203. [Google Scholar] [CrossRef]
- Fukano, Y.; Ito, M. Preparation of GM1 ganglioside with sialidase-producing marine bacteria as a microbial biocatalyst. Appl. Environ. Microbiol. 1997, 63, 1861–1865. [Google Scholar]
- Wang, X.D.; Yin, Z.; Peng, Y.F.; Shen, Y.L.; Wei, D.Z. Highly efficient conversion of polysialoganglioside to GM1 with Brevibacterium casei as a microbial biocatalyst. Biocatal. Biotransform. 2005, 23, 29–32. [Google Scholar] [CrossRef]
- Peng, Y.F.; Wang, X.D.; Wei, D.Z. Development of a large scale process for the conversion of polysialogangliosides to monosialotetrahexosylganglioside with a novel strain of Brevibacterium casei producing sialidase. Biotechnol. Lett. 2007, 29, 885–889. [Google Scholar] [CrossRef]
- Zhang, J.G.; Cao, D.; Shen, D.H.; Wang, X.D.; Wei, D.Z. Efficient conversion from polysialogangliosides to monosialotetrahexosylganglioside using Oerskovia xanthineolytica YZ-2. Bioprocess Biosyst. Eng. 2011, 34, 493–498. [Google Scholar] [CrossRef]
- Scandroglio, F.; Loberto, N.; Valsecchi, M.; Chigorno, V.; Prinetti, A.; Sonnino, S. Thin layer chromatography of gangliosides. Glycoconj. J. 2009, 26, 961–973. [Google Scholar] [CrossRef]
- Gangdini, C.; Kitsos, M.; Massolini, G.; Delorenzi, E.; Soldi, A.; Caccialanza, G.; Kirschner, G. Determination of gangliosides in parenteral dosage form by high-performance liquid chromatography. J. Pharm. Biomed. 1990, 8, 1063–1066. [Google Scholar] [CrossRef]
- Ikeda, K.; Shimizu, T.; Taguchi, R. Targeted analysis of ganglioside and sulfatide molecular species by LC/ESI-MS/MS with theoretically expanded multiple reaction monitoring. J. Lipid Res. 2008, 49, 2678–2689. [Google Scholar] [CrossRef]
- Hildebrandt, H.; Jonas, U.; Ohashi, M.; Klaiber, I.; Rahmann, H. Direct electrospary-ionization mass spectrometric analysis of the major ganglioside from crucian carp liver after thin layer chromatography. Comp. Biochem. Physiol. B 1999, 122, 83–88. [Google Scholar] [CrossRef]
- Pospiech, A.; Neumann, B. A versatile quick-prep of genomic DNA from gram-positive bacteria. Trends Genet. 1995, 11, 217–218. [Google Scholar] [CrossRef]
- Pukin, A.V.; Weijers, C.A.G.M.; Lagen, B.; Wechselberger, R.; Sun, B.; Gilbert, M.; Karwaski, M.F.; Florack, D.E.A.; Jacobs, B.C.; Tio-Gillen, A.P.; et al. GM3, GM2 and GM1 mimics designed for biosensing: Chemoenzymatic synthesis, target affinities and 900 MHz NMR analysis. Carbohydr. Res. 2008, 343, 636–650. [Google Scholar] [CrossRef]
- Ledeen, R.W.; Yu, R.K. Gangliosides: Structure, isolation, and analysis. Methods Enzymol. 1985, 83, 139–191. [Google Scholar]
- Sample Availability: Sample of compound GM1 is available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Ji, L.; Leng, J.; Yuan, Y.; Chen, H.; Gou, D.; Gao, Y.; Zhou, Y. Efficient Biotransformation of Polysialogangliosides for Preparation of GM1 by Cellulosimicrobium sp. 21. Molecules 2014, 19, 16001-16012. https://doi.org/10.3390/molecules191016001
Zheng Y, Ji L, Leng J, Yuan Y, Chen H, Gou D, Gao Y, Zhou Y. Efficient Biotransformation of Polysialogangliosides for Preparation of GM1 by Cellulosimicrobium sp. 21. Molecules. 2014; 19(10):16001-16012. https://doi.org/10.3390/molecules191016001
Chicago/Turabian StyleZheng, Yan, Li Ji, Jiayi Leng, Ye Yuan, Honglei Chen, Dongxia Gou, Yufei Gao, and Yifa Zhou. 2014. "Efficient Biotransformation of Polysialogangliosides for Preparation of GM1 by Cellulosimicrobium sp. 21" Molecules 19, no. 10: 16001-16012. https://doi.org/10.3390/molecules191016001