Bioactivity and Chemical Synthesis of Caffeic Acid Phenethyl Ester and Its Derivatives
Abstract
:1. Introduction

2. Biological Activities
2.1. Anti-Oxidant and Anti-Inflammatory Activities of CAPE
2.2. Immunomodulatory Activity of CAPE
2.3. The Anticancer Activity of CAPE
2.4. Other Biological Activities of CAPE
3. Chemical Synthetic Method
3.1. Using Caffeic Acid as a Substrate for Synthesis of CAPE
3.1.1. Catalyst for Directly Catalysing the Production of CAPE from Caffeic Acid and Phenethyl Alcohol

3.1.2. The Acyl Chloride Method

3.1.3. The Reaction of Caffeic Acid and β-Phenyl Ethyl Bromide

3.2. Using 3,4-Dihydroxy Benzaldehyde as a Substrate for Synthesis of CAPE
3.2.1. Witting Reaction

3.2.2. Malonic Acid Monoester Method

3.2.3. One Pot Method

4. Biosynthetic Method
4.1. Chlorogenic Acid Hydrolase Catalyzed Synthesis



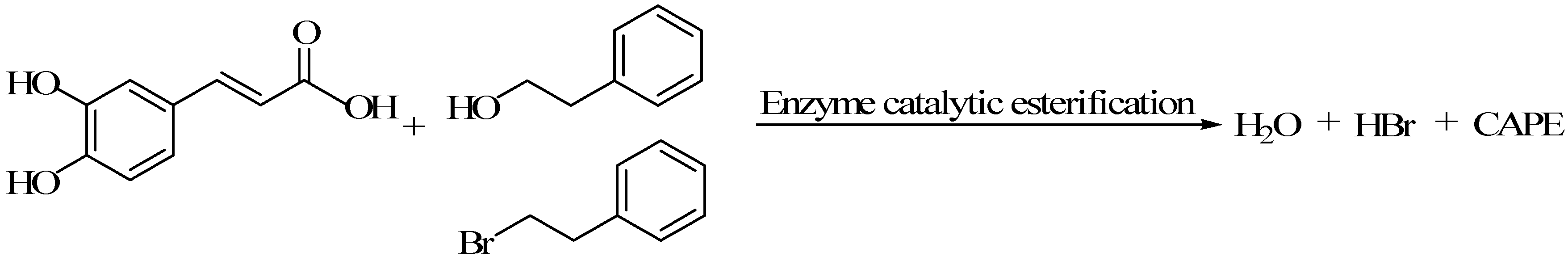
4.2. Lipase Catalyzed Synthesis
5. Synthesis of CAPE Derivatives and Their Structure-Activity Relationships
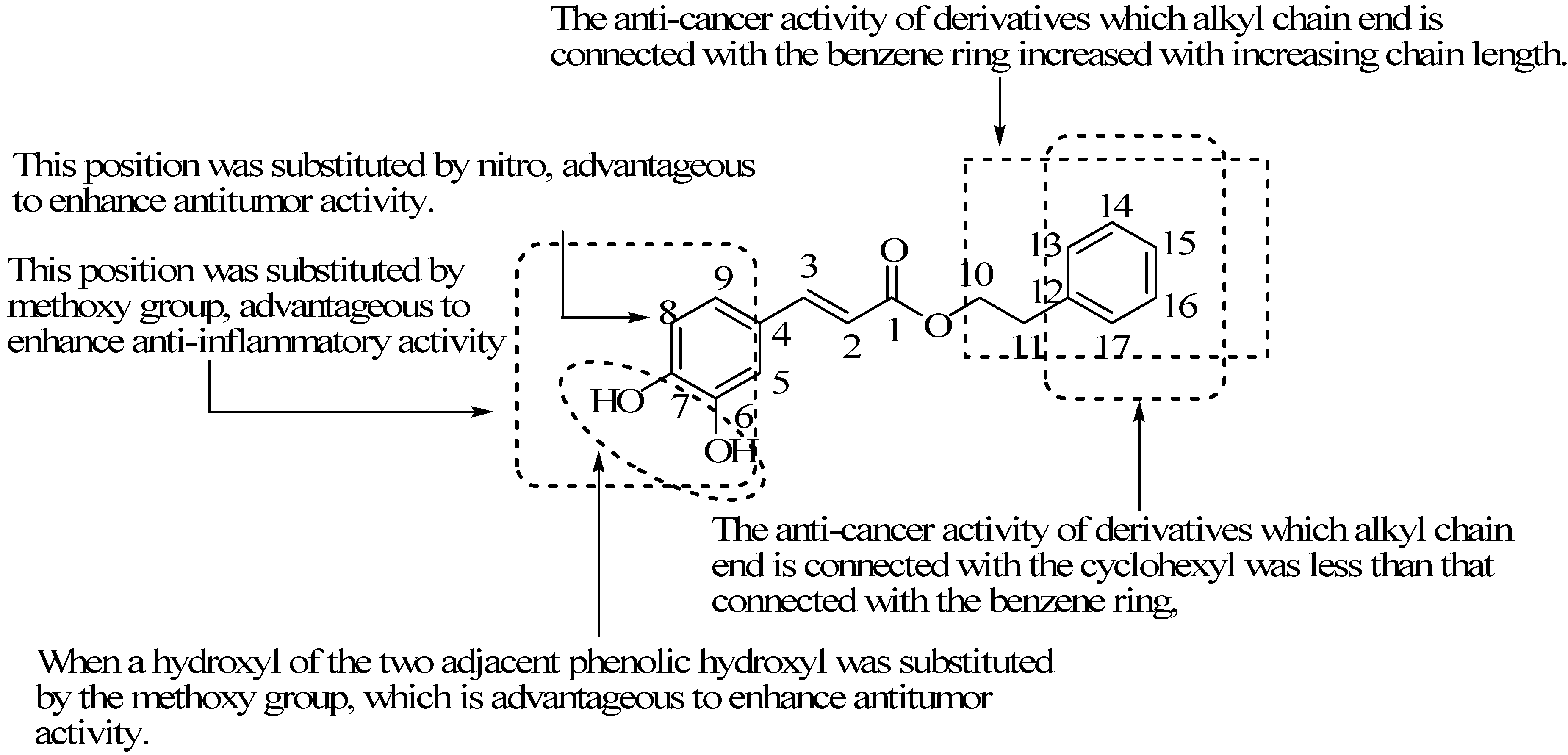
5.1. Two Adjacent Phenolic Hydroxyls Substituted Derivatives


5.2. Benzene Hydrogen Substituted Derivatives


5.3. Alkyl Chain Extended Derivatives


5.4. Caffeic Amide Derivatives
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar]
- Bankova, V.S.; Castro, L.D.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar]
- Chen, Y.J.; Shiao, M.S.; Wang, S.Y. The antioxidant caffeic acid phenethyl ester induces apoptosis associated with selective scavenging of hydrogen peroxide in human leukemic HL-60 cells. Anticancer Drugs 2001, 12, 143–149. [Google Scholar]
- Lee, Y.J.; Liao, P.H.; Chen, W.K.; Yang, C.Y. Preferential cytotoxicity of caffeic acid phenethyl ester analogues on oral cancer cells. Cancer Lett. 2000, 153, 51–56. [Google Scholar]
- Pascual, C.; Gonzales, R.; Torricella, R.G. Scaven gingaction of propolis extract agents oxygen radicals. J. Ethnopharmacol. 1994, 41, 9–13. [Google Scholar]
- Sud’ina, G.F.; Mirzoeva, O.K.; Pushkareva, M.A.; Korshunova, G.A.; Sumbatyan, N.V.; Varfolomeev, S.D. Caffeic acid phenetyl ester as a lipoxygenase inhibitor with antioxidant properties. FEBS Lett. 1993, 329, 21–24. [Google Scholar]
- Krol, W.; Scheller, S.; Czuba, Z.; Matsuno, T.; Zydowicz, G.; Shani, J.; Mos, M. Inhibition of neutrophils’ chemilum inescence by ethanol extract of propolis (EEP) and its phenolic components. J. Ethnopharmacol. 1996, 55, 19–25. [Google Scholar]
- Michaluart, P.; Masferrer, J.L.; Carothrs, A.M.; Subbaramaiah, K.; Zweifel, B.S.; Koboldt, C.; Mestre, J.R.; Grunberqer, D.; Sacks, P.G.; Tanabe, T.; et al. Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res. 1999, 59, 2347–2352. [Google Scholar]
- Park, E.H.; Kahng, J.H. Suppressive effects of propolis in rat adjuvant arthritis. Arch. Pharm. Res. 1999, 22, 554–558. [Google Scholar]
- Ozyurt, H.; Pekmez, H.; Parlaktas, B.S.; Kus, I.; Ozyurt, B.; Sarsilmaz, M. Oxidative stress in testicular tissues of rats exposed to cigarette smoke and protective effects of caffeic acid phenethyl ester. Asian J. Androl. 2006, 8, 189–193. [Google Scholar]
- Gurel, A.; Armutcu, F.; Hosnuter, M.; Unalacak, M.; Kargi, E.; Altinyazar, C. Caffeic acid phenethyl ester improves oxidative organ damage in rat model of thermal trauma. Physiol. Res. 2004, 53, 675–682. [Google Scholar]
- Mirzoeva, O.K.; Calder, P.C. The effect of propolis and its components on eicosanoid product ionduring the inflammatory response. Prostaglandins Leukot. Essent. Fatty Acids 1996, 55, 441–449. [Google Scholar]
- Okutan, H.; Ozcelik, N.; Yilmaz, H.R.; Uz, E. Effects of caffeic acid phenethyl ester on lipid peroxidation and antioxidant enzymes in diabetic rat heart. Clin. Biochem. 2005, 38, 191–196. [Google Scholar]
- Pan, D.; Li, D. Protectiveeffect of caffeicacidphenethylester on myocardialinjury due to anti-oxidantaction. Anadolu Kardiyol. Derg 2014, 14. [Google Scholar] [CrossRef]
- Kim, J.K.; Jang, H.D. Nrf2-mediated HO-1 induction coupled with the ERK signaling pathway contributes to indirect antioxidant capacity of caffeic acid phenethyl ester in HepG2 cells. Int. J. Mol. Sci. 2014, 15, 12149–12165. [Google Scholar]
- Yang, J.W.; Jung, W.K.; Lee, C.M.; Yea, S.S.; Choi, Y.H.; Kim, G.Y.; Lee, D.S.; Na, G.; Park, S.G.; Seo, S.K.; et al. Caffeic acid phenethyl ester inhibits the inflammatory effects of interleukin-1β in human corneal fibroblasts. Immunopharmacol. Immunotoxicol. 2014, 36, 371–377. [Google Scholar]
- Cho, M.S.; Park, W.S.; Jung, W.K.; Qian, Z.J.; Lee, D.S.; Choi, J.S.; Lee, D.Y.; Park, S.G.; Seo, S.K.; Kim, H.J.; et al. Caffeic acid phenethyl ester promotes anti-inflammatory effects by inhibiting MAPK and NF-κB signaling in activated HMC-1 human mast cells. Pharm. Biol. 2014, 52, 926–932. [Google Scholar]
- Zhao, W.X.; Wang, L.; Yang, J.L.; Li, L.Z.; Li, T. Caffeic acid phenethyl ester attenuates pro-inflammatoryand fibrogenic phenotypes of LPS-stimulated hepatic stellatecells through the inhibition of NF-κB signaling. Int. J. Mol. Med. 2014, 33, 687–694. [Google Scholar]
- Lee, K.W.; Chun, K.S.; Lee, J.S.; Kang, K.S.; Surh, Y.J.; Lee, H.J. Inhibition of cyclooxygenase-2 expression and restoration of gap junction intercellular communication in H-ras-transformed rat liver epithelial cells by ca eic acid phenethyl ester. Ann. N. J. Acad. Sci. 2004, 103, 501–507. [Google Scholar]
- Akyol, S.; Acar, M.; Unal, Z.N.; Hasgul, R. The effects of caffeic acid phenethyl ester (CAPE), royal jelly, and curcumin on gene expression of ADAMTS-1, -5, and -9 in OUMS-27 chondrosarcoma cells: A preliminary study. Ann. Paediatr. Rheum. 2013, 2, 27–37. [Google Scholar]
- Lee, J.Y.; Choi, H.J.; Chung, T.W.; Kim, C.H.; Jeong, H.S.; Ha, K.T. Caffeic acid phenethyl ester inhibits alpha-melanocyte stimulating hormone-induced melanin synthesis through suppressing transactivation activity of microphthalmia-associated transcription factor. J. Nat. Prod. 2013, 76, 1399–1405. [Google Scholar]
- Chuu, C.P.; Lin, H.P.; Ciaccio, M.F.; Kokontis, J.M.; Hause, R.J.; Hiipakka, R.A.; Liao, S.; Jones, R.B. Caffeic acid phenethyl ester suppresses the proliferation of human prostate cancer cells through inhibition of p70S6K and Akt signaling networks. Cancer Prev. Res. 2012, 5, 788–797. [Google Scholar]
- Chen, Y.J.; Shiao, M.S.; Hsu, M.L.; Tsai, T.H.; Wang, S.Y. Effects of caffeic acid phenethyl ester, an antioxidant from propolis in human leukemic HL-60 cells. J. Agric. Food Chem. 2001, 49, 5615–5619. [Google Scholar]
- Matsuda, H.; Morikawa, T.; Ando, S.; Toguchida, I.; Yoshikawa, M. Structural requirements of flavonoids for nitric oxide production inhibitory activity and mechanism of action. Bioorg. Med. Chem. 2003, 11, 1995–2000. [Google Scholar]
- Kageura, T.; Matsuda, H.; Morikawa, T.; Toquchida, I.; Harima, S.; Oda, M.; Yoshikawa, M. Inhibitors from rhubarb on lipopolysaccharide- induced nitric oxide production in macrophages: Structural requirements of stilbenes for the Activity. Bioorg. Med. Chem. 2001, 9, 1887–1893. [Google Scholar]
- Matsuda, H.; Morikawa, T.; Ando, S.; Ominami, H.; Murakami, T.; Kimural, I.; Yoshikawa, M. Absolute stereostructures of polypodane-and octanordammarane-type triterpenes with nitric oxide production inhibitory activity from guggul-gum resins. Bioorg. Med. Chem. 2004, 12, 3037–3046. [Google Scholar]
- Song, Y.S.; Park, E.H.; Hur, G.M.; Ryu, Y.S.; Lee, Y.S.; Lee, J.Y.; Kim, Y.M.; Jin, C. Caffeic acid phenethyl ester inhibitsnitric oxide synthase gene expression and enzyme activity. Cancer Lett. 2002, 175, 53–61. [Google Scholar]
- Zhang, X.; Neamati, N.; Lee, Y.K.; Orr, A.; Brown, R.D.; Whitaker, N.; Pommier, Y.; Burke, T.R. Arylisothiocyanate-containing esters of caffeic acid designed as affinity ligands for HIV-1 integrase. Bioorg. Med. Chem. 2001, 9, 1649–1657. [Google Scholar]
- Nomura, M.; Kaji, A.; Ma, W.; Miyamoto, K.; Dong, Z. Suppression of cell transformation and induction of apoptosis by caffeic acid phenethyl ester. Mol. Carcinog. 2001, 31, 83–89. [Google Scholar]
- Costi, R.; Santo, R.D.; Artico, M.; Massa, S.; Ragno, R.; Loodo, R.; La, C.M.; Tramontano, E.; La, C.P.; Pani, A. 2,6-Bis(3,4,5-trihydroxybenzylydene) derivatives of cyclohexanone: Novel potent HIV-1 integrase inhibitors that prevent HIV-1 multiplication in cell-based assays. Bioorg. Med. Chem. 2004, 12, 199–215. [Google Scholar]
- Natarajan, K.; Singh, S.; Burke, T.R.; Grunberger, D.; Aggarwal, B.B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc. Natl. Acad. Sci. USA 1996, 93, 9090–9095. [Google Scholar]
- De Clercq, E. Non-nucleoside reverse transcriptase inhibitors (NNRTIs) for the treatment of huamn immunodeficiency virus type1 (HIV-1) infections: Strategies to overcome drug resistance development. Med. Res. Rev. 1996, 16, 125–157. [Google Scholar]
- Mahmoud, N.N.; Carothers, A.M.; Grunberger, D.; Bilinski, R.T.; Churchill, M.R.; Martucci, C.; Newmark, H.L.; Bertaqnoli, M.M. Plant phenolics decrease intestinal tumors in an animalmodel of familial adenomatous polyposis. Carcinogenesis 2000, 21, 921–927. [Google Scholar]
- Fiuza, S.M.; Gomes, C.; Teixeira, L.J.; Cruz, M.T.; Cordeiro, M.N.; Milhazes, N.; Marques, M.P. Phenolic acid derivatives with potential anticancer properties—A structure-activity relationship study. Part 1: Methyl, propyl and octyl esters of caffeic and gallic acids. Bioorg. Med. Chem. 2004, 12, 3581–3589. [Google Scholar]
- Akyol, S.; Ozturk, G.; Ginis, Z.; Armutcu, F.; Yiqitoqlu, M.R.; Akyol, O. In vivo and in vitro antineoplastic actions of caffeic acid phenethyl ester (CAPE): Therapeutic perspectives. Nutr. Cancer 2013, 65, 515–526. [Google Scholar]
- Bankova, V.S.; Popov, S.S.; Marekov, N.L. A study on flavonoids of propolis. J. Nat. Prod. 1983, 46, 471–474. [Google Scholar]
- Brumfitt, W.; Hamilton-Miller, M.J.; Franklin, I. Antibiotic activity of natural product: 1. Propolis. Microbios 1990, 62, 19–22. [Google Scholar]
- Tolba, M.F.; Azab, S.S.; Khalifa, A.E. Caffeic acid phenethyl ester, a promising component of propolis with a plethora of biological activities: A review on its anti-inflammatory, neuroprotective, hepatoprotcvtive and cardioprotective. IUBMB Life 2013, 65, 699–709. [Google Scholar]
- Ozturk, G.; Ginis, Z.; Akyol, S.; Erden, G.; Gurel, A.; Akyol, O. The anticancer mechanism of caffeic acid phenethyl ester (CAPE): Review of melanomas, lung and prostate cancers. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 2064–2068. [Google Scholar]
- Eisinger, M.; Marko, O.; Ogata, S.; Old, L.J. Growth regulation of human melanocytes mitogenic factor in extracts of melanomn, astrocytoma, and fibrolast cell lines. Science 1985, 229, 984–986. [Google Scholar]
- Hwang, H.J.; Park, H.J.; Chung, H.J.; Min, H.Y.; Park, E.J.; Hong, J.Y.; Lee, S.K. Inhibitory effects of caffeic acid phenethyl ester on cancer cell metastasis mediated by the down-regulation of matrix metalloproteinase expression in human HT1080 fibrosarcoma cells. J. Nutr. Biochem. 2006, 17, 356–362. [Google Scholar]
- Grunberger, D.; Banerjee, R.; Eisinger, K.; Oltz, E.M.; Efros, L.; Caldwell, M.; Estevez, V.; Nakanishi, K. Preferential cytotoxicity on tumor cell by caffeic acid phenethyl ester isolated from Propolis. Experientia 1988, 44, 230–232. [Google Scholar]
- Lin, W.L.; Liang, W.H.; Lee, Y.J.; Chung, S.K.; Tseng, T.H. Antitumor progression potential of caffeic acid phenethyl ester involving p75(NTR) in C6 glioma cells. Chem. Biol. Interact. 2010, 188, 607–615. [Google Scholar]
- Chiao, C.; Carothers, A.M.; Grunberger, D.; Solomon, G.; Preston, G.A.; Barrett, J.C. Apotosis and altered redox state induced by caffeic acid phenethyl ester (CAPE) in transformed rat fibroblast cells. Cancer Res. 1995, 55, 3576–3583. [Google Scholar]
- Nagaoka, T.; Banskota, A.H.; Tezuka, Y.; Saiki, I.; Kadada, S. Selective antiproliferative activity of caffeic acid phenethyl ester analogues on highly liver-metastatic murine colon 26-L5 carcinoma cell line. Bioorg. Med. Chem. 2002, 10, 3351–3359. [Google Scholar]
- Onori, P.; DeMorrow, S.; Gaudio, E.; Franchitto, A.; Mancinelli, R.; Venter, J.; Kopriva, S.; Ueno, Y.; Alvaro, D.; Savage, J.; et al. Caffeic acid phenethyl ester decreases cholangiocarcinoma growth by inhibition of NF-κB and induction of apoptosis. Int. J. Cancer 2009, 125, 565–576. [Google Scholar]
- Chiang, E.P.; Tsai, S.Y.; Kuo, Y.H.; Pai, M.H.; Liu, H.L.; Rodriguez, R.L.; Tang, F.Y. Caffeic acid derivatives inhibit the growth of colon cancer: Involvement of the PI3-K/Akt and AMPK signaling pathways. PLoS One 2014, 9, e99631. [Google Scholar]
- Chen, M.F.; Lu, M.S.; Chen, P.T.; Chen, W.C.; Lin, P.Y.; Lee, K.D. Role of interleukin 1beta in esophageal squamous cell carcinoma. J. Mol. Med. 2012, 90, 89–100. [Google Scholar]
- Wu, J.; Omene, C.; Karkoszka, J.; Bosland, M.; Eckard, J.; Klein, C.B.; Frenkel, K. Caffeic acid phenethyl ester (CAPE), derived from a honeybee product propolis, exhibitsa diversity of anti-tumor effects in pre-clinical models of human breastcancer. Cancer Lett. 2011, 308, 43–53. [Google Scholar]
- Omene, C.O.; Wu, J.; Frenkel, K. Caffeic acid phenethyl ester (CAPE) derived from propolis, a honeybee product, inhibits growth of breast cancer stem cells. Investig. New Drugs 2012, 30, 1279–1288. [Google Scholar]
- Ozer, M.K.; Parlakpinar, H.; Acet, A. Reduction of ischemia-reperfusion induced myocardial infarct size in rats by caffeic acid phenethyl ester (CAPE). Clin. Biochem. 2004, 37, 702–705. [Google Scholar]
- Motor, S.; Alp, H.; Senol, S.; Pinar, N.; Moter, V.K.; Kaplan, L.; Alp, A.; Gokce, C. Comparison of the chronic effects of ribavirin and caffeic acid phenethyl ester (CAPE) on pancreatic damage and hepatotoxicity. Int. J. Clin. Exp. Med. 2014, 15, 1005–1013. [Google Scholar]
- Francesca, B.; Francesco, C. CAPE, the composition of propolis. Science 2003, 24, 60–70. [Google Scholar]
- Chen, J.H.; Shao, Y.; Huang, M.T.; Chin, C.K.; Ho, C.T. Inibitory effect of caffeic acid Phenethyl ester on human leukemia HL-60 cells. Cancer Lett. 1996, 1108, 221–214. [Google Scholar]
- Son, S.; Lobkowsky, E.B.; Lewis, B.A. Caffeic acid phenethyl ester (CAPE): Synthesis and X-ray crystal lographic analysis. Chem. Pharm. Bull. 2001, 49, 236–238. [Google Scholar]
- Chen, W.K.; Tsai, C.F.; Liao, P.H.; Kuo, S.C.; Lee, Y.J. Synthesis of caffeic acid esters as antioxidants by esterification via acyl chlorides. Chin. Pharm. J. 1999, 51, 271–278. [Google Scholar]
- Bankova, V.S. Synthesis of natural esters of substituted cinnamic acid. J. Nat. Prod. 1990, 53, 821–824. [Google Scholar]
- Boutagy, J.; Thomas, R. Olefin synthesis with organic phosphonate carbanions. Chem. Rev. 1974, 74, 87–99. [Google Scholar]
- Piechucki, C. Phase-transfer catalyzed wittig-horner reactions of diethyl phenyl and styryl methan ephosphonates: A simple preparation of 1-aryl-4-phenylbuta-1, 3-dienes. Synthesis 1976, 3, 187–188. [Google Scholar]
- Xia, C.; Hu, W. Synthesis of caffeic acid esters. J. Chem. Res. 2005, 5, 332–334. [Google Scholar]
- Ryu, Y.; Scott, A.I. Self-condensation of activated malonic acid half esters: A model for the decarboxylative claisen condensation in polyketide biosynthesis. Tetrahedron Lett. 2003, 44, 7499–7502. [Google Scholar]
- Kishimoto, N.; Kakino, Y.; Iwai, K. Enzymatic synthesis of caffeic acid esters from chlorogenic acid by transesterification and condensation reactions. Colloq. Sci. Int. Cafe 2005, 20, 249–253. [Google Scholar]
- Kishimoto, N.; Kakino, Y.; Iwai, K. Chlorogenate hydrolase-catalyzed synthesis of hydroxycinnamic acid ester derivatives by transesterification, substitution of bromine, and condensation reactions. Appl. Microbiol. Biotechnol. 2005, 68, 198–202. [Google Scholar]
- Stevenson, D.E.; Parkar, S.G.; Zhang, J.; Stanley, R.A.; Jensen, D.J.; Cooney, J.M. Combinatorial enzymic synthesis for functional testing of phenolic acid esters catalysed by Candida antarctica lipase B (Novozym 435(R)). Enzyme Microb. Technol. 2006, 40, 1078–1086. [Google Scholar]
- Widjaja, A.; Yeh, T.; Ju, Y. Enzymatic synthesis of caffeic acid phenethyl ester. J. Chin. Inst. Chem. Eng. 2008, 39, 413–418. [Google Scholar]
- Lee, Y.T.; Don, M.J.; Hung, P.S.; Shen, Y.C.; Lo, Y.S.; Chang, K.W.; Chen, C.F.; Ho, L.K. Cytotoxicty of phenolic acid phenethyl esters on oral cancer cells. Cancer Lett. 2004, 223, 19–25. [Google Scholar]
- Wang, X.; Stavchansky, S.; Bowman, P.D.; Kerwin, S.M. Cytoprotective effect of caffeic acid phenethyl ester (CAPE) and catechol ring-fluorinated CAPE derivatives against menadione-induced oxidative stress in human endothelial cells. Bioorg. Med. Chem. 2006, 14, 4879–4887. [Google Scholar]
- Xia, C.N.; Li, H.B.; Liu, F.; Hu, W.X. Synthesis of trans-caffeate analogues and their bioactivities against HIV-1 integrase and cancer cell lines. Bioorg. Med. Chem. Let. 2008, 18, 6553–6557. [Google Scholar]
- Nagaoka, T.; Banskota, A.H.; Tezuka, Y.; Harimaya, Y.; Koizumi, K.; Saiki, I.; Kadota, S. Inhabitory effects of caffeic acid phenethyl ester analogues on experimental lung metastasis of murine colon 26-L5 carcinoma cells. Biol. Pharm. Bull. 2003, 26, 638–641. [Google Scholar]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Tang, Y.; Li, N.-G.; Zhu, Y.; Duan, J.-A. Bioactivity and Chemical Synthesis of Caffeic Acid Phenethyl Ester and Its Derivatives. Molecules 2014, 19, 16458-16476. https://doi.org/10.3390/molecules191016458
Zhang P, Tang Y, Li N-G, Zhu Y, Duan J-A. Bioactivity and Chemical Synthesis of Caffeic Acid Phenethyl Ester and Its Derivatives. Molecules. 2014; 19(10):16458-16476. https://doi.org/10.3390/molecules191016458
Chicago/Turabian StyleZhang, Pengxuan, Yuping Tang, Nian-Guang Li, Yue Zhu, and Jin-Ao Duan. 2014. "Bioactivity and Chemical Synthesis of Caffeic Acid Phenethyl Ester and Its Derivatives" Molecules 19, no. 10: 16458-16476. https://doi.org/10.3390/molecules191016458
APA StyleZhang, P., Tang, Y., Li, N.-G., Zhu, Y., & Duan, J.-A. (2014). Bioactivity and Chemical Synthesis of Caffeic Acid Phenethyl Ester and Its Derivatives. Molecules, 19(10), 16458-16476. https://doi.org/10.3390/molecules191016458



