Evaluation of the Toxicity of 5-Aryl-2-Aminoimidazole-Based Biofilm Inhibitors against Eukaryotic Cell Lines, Bone Cells and the Nematode Caenorhabditis elegans
Abstract
:1. Introduction


2. Results and Discussion
2.1. Cytostatic Activity against Tumor Cell Lines

| IC50a (µM) | BIC50b (µM) | TI cS. Typhimurium | TI P. aeruginosa | C. elegans | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n° | R4 | R1 | R2 | R3 | L1210 | CEM | HeLa | S. Typhimurium | P. aeruginosa | L1210 | CEM | HeLa | L1210 | CEM | HeLa | % survival at 25 µM e | |||
| monosubstituted | |||||||||||||||||||
| 1 | H | H | H | H | 62.9 | 94.3 | 144.6 | 130.2 | 72.6 | 0.5 | 0.7 | 1.1 | 0.9 | 1.3 | 2.0 | 89.8 | |||
| 2 | 4-Cl | H | H | H | 77.5 | 49.6 | 72.3 | 16 | 3.5 | 4.8 | 3.1 | 4.5 | 22.1 | 14.2 | 20.7 | 84.9 | |||
| 3 | 4-Ph | H | H | H | 37.4 | 13.6 | 34.0 | 17.3 | 8.6 | 2.2 | 0.8 | 2.0 | 4.3 | 1.6 | 4.0 | nd | |||
| 4 | 4-NO2 | H | H | H | 83.3 | 63.7 | 107.7 | 17.6 | 34.5 | 4.7 | 3.6 | 6.1 | 2.4 | 1.8 | 3.1 | 97.3 | |||
| 5 | 4-Br | H | H | H | 10.1 | 13.9 | 21.8 | 47.9 | 3.2 | 0.2 | 0.3 | 0.5 | 3.2 | 4.3 | 6.8 | 90.6 | |||
| N1-substituted | |||||||||||||||||||
| 6 | H | Oct | H | H | 3.2 | 467.9 | 3.0 | 11.9 | 18.5 | 0.3 | 39.3 | 0.3 | 0.2 | 25.3 | 0.2 | 92.5 | |||
| 7 | 4-Cl | Oct | H | H | 3.9 | 356.8 | 4.5 | 5.9 | 4.0 | 0.7 | 60.5 | 0.8 | 1.0 | 89.2 | 1.1 | 86.6 | |||
| 8 | 4-Cl | Trd | H | H | 7.2 | 6.4 | 4.5 | 19.5 | 33.9 | 0.4 | 0.3 | 0.2 | 0.2 | 0.2 | 0.1 | nd | |||
| 9 | 4-Ph | Hept | H | H | 7.5 | 8.1 | 8.7 | 9.3 | 25.2 | 0.8 | 0.9 | 0.9 | 0.3 | 0.3 | 0.3 | nd | |||
| 10 | 4-Ph | Oct | H | H | 7.8 | 8.3 | 6.6 | 3.3 | 8.9 | 2.4 | 2.5 | 2.0 | 0.9 | 0.9 | 0.7 | nd | |||
| 11 | 4-Ph | c-Pen | H | H | 4.3 | 2.9 | 8.2 | 7.5 | 353.1 | 0.6 | 0.4 | 1.1 | 0.01 | 0.01 | 0.02 | nd | |||
| 12 | 4-Ph | c-Hept | H | H | 8.1 | 5.4 | 6.6 | 12.5 | 6.6 | 0.6 | 0.4 | 0.5 | 1.2 | 0.8 | 1.0 | nd | |||
| 13 | 4-Ph | c-Oct | H | H | 6.9 | 4.9 | 6.9 | 22.9 | 3.2 | 0.3 | 0.2 | 0.3 | 2.2 | 1.5 | 2.2 | nd | |||
| 14 | 4-F | Oct | H | H | 3.5 | 7.3 | 8.6 | 10.8 | 4.9 | 0.3 | 0.7 | 0.8 | 0.7 | 1.5 | 1.8 | nd | |||
| 15 | 4-SMe | Oct | H | H | 4.1 | 7.2 | 6.3 | 6.7 | 2.8 | 0.6 | 1.1 | 0.9 | 1.5 | 2.6 | 2.3 | nd | |||
| 16 | 3-Br | Oct | H | H | 6.9 | 9.7 | 8.0 | 11.2 | 40.6 | 0.6 | 0.9 | 0.7 | 0.2 | 0.2 | 0.2 | nd | |||
| 17 | 3,4-diCl | m-MeOPhenethyl | H | H | 6.9 | 8.6 | 6.6 | >400 | nd d | <0.02 | <0.02 | <0.02 | nd | nd | nd | nd | |||
| 2N-substituted | |||||||||||||||||||
| 18 | H | H | Bu | H | 46.4 | 667.4 | 19.5 | 25.3 | 31.8 | 1.8 | 26.4 | 0.8 | 1.5 | 21.0 | 0.6 | nd | |||
| 19 | H | H | i-Bu | H | 83.6 | 382.7 | 91.4 | 4.9 | 1.2 | 17.1 | 78.1 | 18.7 | 69.7 | 318.9 | 76.2 | nd | |||
| 20 | H | H | c-Pen | H | 27.3 | 293.3 | 27.3 | 52.9 | 33.8 | 0.5 | 5.5 | 0.5 | 0.8 | 8.7 | 0.8 | 98.1 | |||
| 21 | 4-Cl | H | Bu | H | 40.0 | 374.6 | 34.7 | nd | nd | nd | nd | nd | nd | nd | nd | nd | |||
| 22 | 4-Cl | H | i-Bu | H | 336.3 | 297.3 | 171.5 | 2 | 0.9 | 168.2 | 148.7 | 85.8 | 373.7 | 330.3 | 190.6 | 97.6 | |||
| 23 | 4-Cl | H | Pen | H | 22.0 | 322.9 | 19.8 | >400 | 6.3 | <0.05 | <0.8 | <0.05 | 3.5 | 51.3 | 3.1 | nd | |||
| 24 | 4-Cl | H | c-Pen | H | 35.5 | 591.0 | 28.8 | 4.4 | 13.5 | 8.1 | 134.3 | 6.5 | 2.6 | 43.8 | 2.1 | 100.1 | |||
| 25 | 4-Br | H | Bu | H | 125.8 | 493.0 | 50.7 | 7.1 | 9.8 | 17.7 | 69.4 | 7.1 | 12.8 | 50.3 | 5.2 | 102.4 | |||
| 26 | 4-Br | H | i-Bu | H | 31.7 | 306.0 | 22.6 | 2.9 | 1.2 | 10.9 | 105.5 | 7.8 | 26.4 | 255.0 | 18.8 | nd | |||
| 27 | 4-Br | H | Pen | H | 14.9 | 375.2 | 16.3 | 3.1 | 10.2 | 4.8 | 121.0 | 5.2 | 1.5 | 36.8 | 1.6 | nd | |||
| 28 | 4-Br | H | c-Pen | H | 32.7 | 673.6 | 32.7 | 12.1 | 7.2 | 2.7 | 55.7 | 2.7 | 4.5 | 93.6 | 4.5 | nd | |||
| 29 | 3,4-diCl | H | c-Pen | H | 76.1 | 155.9 | 79.8 | 5.7 | 7.9 | 13.4 | 27.4 | 14.0 | 9.6 | 19.7 | 10.1 | nd | |||
| 4,5-disubstituted | |||||||||||||||||||
| 30 | H | H | H | 4-OMePh | 64.1 | 52.8 | 45.2 | 77.1 | nd | 0.8 | 0.7 | 0.6 | nd | nd | nd | nd | |||
| 31 | H | H | H | 4-ClPh | 59.3 | 48.2 | 40.8 | 46.9 | nd | 1.3 | 1.0 | 0.9 | nd | nd | nd | nd | |||
| 32 | 4-Cl | H | H | 4-MePh | 12.3 | 10.2 | 9.5 | 12.9 | nd | 1.0 | 0.8 | 0.7 | nd | nd | nd | nd | |||
| 33 | 4-Cl | H | H | 4-CF3Ph | 41.5 | 35.5 | 26.1 | 10.8 | nd | 3.8 | 3.3 | 2.4 | nd | nd | nd | nd | |||
| 34 | 4-F | H | H | amidophenyl | 74.2 | 74.2 | 77.6 | 182.1 | nd | 0.4 | 0.4 | 0.4 | nd | nd | nd | nd | |||
| 1,4,5-trisubstituted | |||||||||||||||||||
| 35 | 4-Me | Ben | H | p-PenOBn | 8.6 | 8.4 | 14.8 | 10.3 | 27.4 | 0.8 | 0.8 | 1.4 | 0.3 | 0.3 | 0.5 | nd | |||
| 2AI-triazole conjugates | |||||||||||||||||||
| 36 | H | H | n = 2, R'= Ph | H | 221.0 | 452.6 | 72.9 | 36.5 | >400 | 6.1 | 12.4 | 2.0 | <0.6 | <1.1 | <0.2 | 87.7 | |||
| 37 | H | H | n = 3, R' = Ph | H | 217.8 | 459.2 | 106.7 | 40.0 | 19.0 | 5.4 | 11.5 | 2.7 | 11.5 | 24.2 | 5.6 | 86.4 | |||
| 38 | 4-OMe | H | n = 2, R' = 4-BrPh | H | 18.4 | 596.6 | 16.8 | 91.2 | 42.7 | 0.2 | 6.5 | 0.2 | nd | nd | nd | nd | |||
| 39 | 4-Br | H | n = 2, R' = c-Hex | H | 20.7 | 396.0 | 22.5 | 8.4 | 12.5 | 2.5 | 47.1 | 2.7 | 1.7 | 31.7 | 1.8 | 99.3 | |||
| 40 | 4-Br | H | n = 2, R' = c-Pr | H | 43.9 | 33.6 | 67.1 | 2.0 | 71.6 | 21.9 | 16.8 | 33.6 | 0.6 | 0.5 | 0.9 | 82.6 | |||


2.2. Effects on Viability and Functional Behavior of Bone Cells

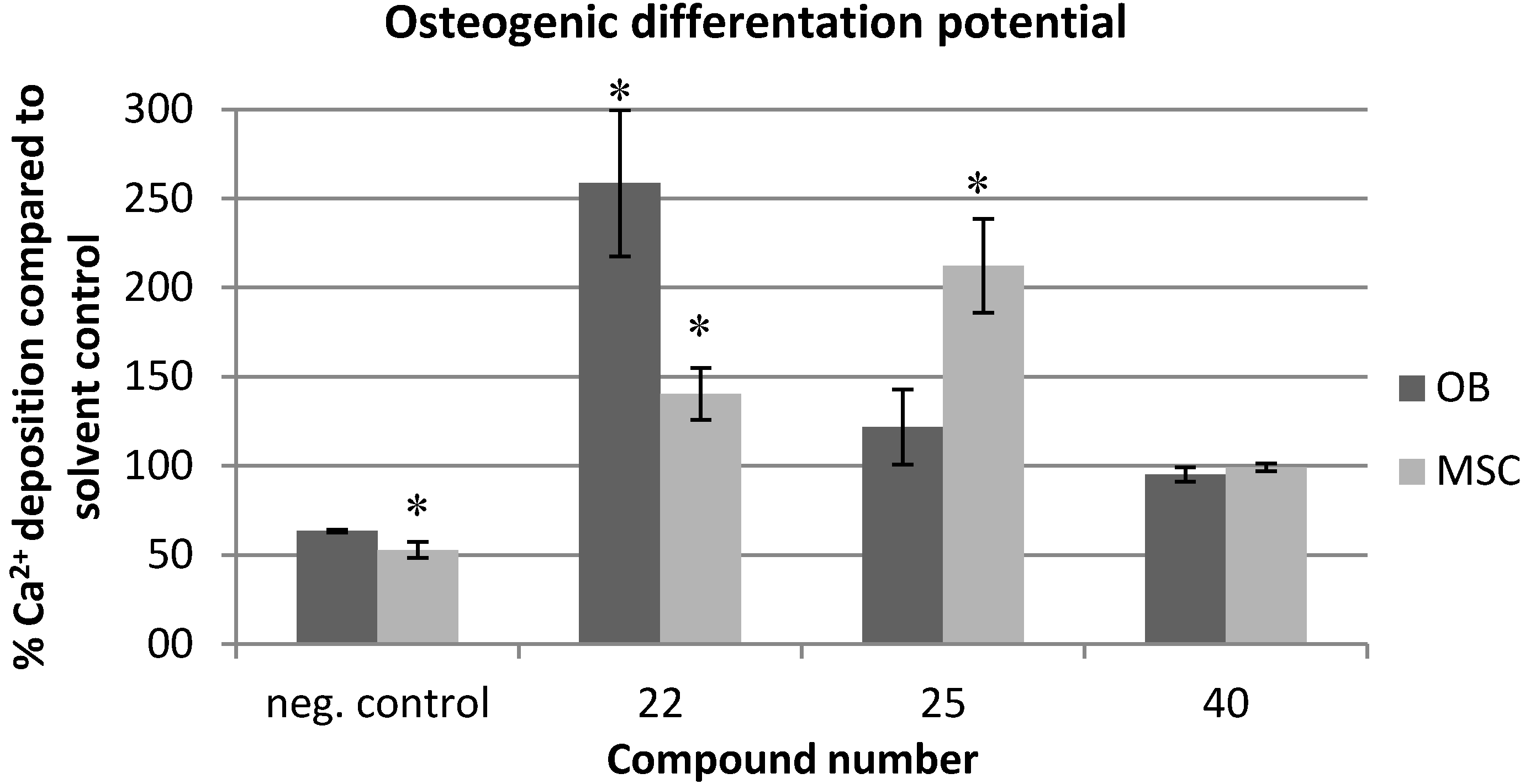
2.3. Evaluation of the Toxicity against C. elegans

3. Experimental Section
3.1. Compound Preparation
3.2. Cytostatic Activity against Tumor Cell Lines
3.3. Cytotoxicity against Osteoblasts and Mesenchymal Stem Cells
3.4. Calcium Assay for Determination of Osteogenic Differentiation Potential
3.5. C. elegans Toxicity Test
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar]
- Hall-Stoodley, L.; Hu, F.Z.; Gieseke, A.; Nistico, L.; Nguyen, D.; Hayes, J.; Forbes, M.; Greenberg, D.P.; Dice, B.; Burrows, A.; et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 2006, 296, 202–211. [Google Scholar]
- Bjarnsholt, T.; Jensen, P.O.; Fiandaca, M.J.; Pedersen, J.; Hansen, C.R.; Andersen, C.B.; Pressler, T.; Givskov, M.; Hoiby, N. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr. Pulmonol. 2009, 44, 547–558. [Google Scholar]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar]
- Hall-Stoodley, L.; Stoodley, P. Evolving concepts in biofilm infections. Cell Microbiol. 2009, 11, 1034–1043. [Google Scholar]
- Hoiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar]
- Jensen, P.O.; Givskov, M.; Bjarnsholt, T.; Moser, C. The immune system vs. Pseudomonas aeruginosa biofilms. FEMS Immunol. Med. Microbiol. 2010, 59, 292–305. [Google Scholar]
- Burmolle, M.; Thomsen, T.R.; Fazli, M.; Dige, I.; Christensen, L.; Homoe, P.; Tvede, M.; Nyvad, B.; Tolker-Nielsen, T.; Givskov, M.; et al. Biofilms in chronic infections—A matter of opportunity—monospecies biofilms in multispecies infections. FEMS Immunol. Med. Microbiol. 2010, 59, 324–336. [Google Scholar]
- Hassett, D.J.; Korfhagen, T.R.; Irvin, R.T.; Schurr, M.J.; Sauer, K.; Lau, G.W.; Sutton, M.D.; Yu, H.; Hoiby, N. Pseudomonas aeruginosa biofilm infections in cystic fibrosis: Insights into pathogenic processes and treatment strategies. Expert Opin. Ther. Targets 2010, 14, 117–130. [Google Scholar]
- Bjarnsholt, T.; Tolker-Nielsen, T.; Hoiby, N.; Givskov, M. Interference of Pseudomonas aeruginosa signalling and biofilm formation for infection control. Expert Rev. Mol. Med. 2010, 12, e11. [Google Scholar]
- Landini, P.; Antoniani, D.; Burgess, J.G.; Nijland, R. Molecular mechanisms of compounds affecting bacterial biofilm formation and dispersal. Appl. Microbiol. Biotechnol. 2010, 86, 813–823. [Google Scholar]
- Steenackers, H.; Ermolat’ev, D.S.; Savaliya, B.; de Weerdt, A.; de Coster, D.; van der Eycken, E.; de Vos, D.; Vanderleyden, J.; de Keersmaecker, S.C. Structure Activity Relationship of 4(5)-Phenyl-2-amino-1H-imidazoles, N1-Substituted 2-Aminoimidazoles and Imidazo[1,2-a]pyrimidinium Salts as Inhibitors of the Biofilm Formation by Salmonella Typhimurium and Pseudomonas aeruginosa. J. Med. Chem. 2010, 54, 472–482. [Google Scholar]
- Steenackers, H.P.; Ermolat’ev, D.S.; Savaliya, B.; Weerdt, A.D.; Coster, D.D.; Shah, A.; van der Eycken, E.V.; de Vos, D.E.; Vanderleyden, J.; de Keersmaecker, S.C. Structure-activity relationship of 2-hydroxy-2-aryl-2,3-dihydro-imidazo[1,2-a]pyrimidinium salts and 2N-substituted 4(5)-aryl-2-amino-1H-imidazoles as inhibitors of biofilm formation by Salmonella Typhimurium and Pseudomonas aeruginosa. Bioorg. Med. Chem. 2011, 19, 3462–3473. [Google Scholar]
- Ermolat’ev, D.S.; Bariwal, J.B.; Steenackers, H.P.; de Keersmaecker, S.C.; van der Eycken, E.V. Concise and diversity-oriented route toward polysubstituted 2-aminoimidazole alkaloids and their analogues. Angew. Chem. Int. Ed. Engl. 2010, 49, 9465–9468. [Google Scholar]
- Steenackers, H.; Ermolat’ev, D.; Trang, T.; Savalia, B.; de Weerdt, A.; Shah, A.; Vanderleyden, J.; van der Eycken, E. Microwave-Assisted One-Pot Synthesis and Anti-Biofilm Activity of 2-Amino-1H-imidazole/Triazole Conjugates. Org. Biomol. Chem. 2014, 12, 3671–3678. [Google Scholar]
- Stowe, S.D.; Richards, J.J.; Tucker, A.T.; Thompson, R.; Melander, C.; Cavanagh, J. Anti-biofilm compounds derived from marine sponges. Mar. Drugs 2011, 9, 2010–2035. [Google Scholar]
- Sullivan, J.D.; Giles, R.L. 2-Aminoimidazoles from Leucetta sponges: Synthesis and Biology of an Important Pharmacophore. Curr. Bioact. Compd. 2009, 5, 39–78. [Google Scholar]
- Lavelle, F.; Zerial, A.; Fizames, C.; Rabault, B.; Curaudeau, A. Antitumor activity and mechanism of action of the marine compound girodazole. Investig. New Drugs 1991, 9, 233–244. [Google Scholar]
- Tsukamoto, S.; Yamashita, K.; Tane, K.; Kizu, R.; Ohta, T.; Matsunaga, S.; Fusetani, N.; Kawahara, H.; Yokosawa, H. Girolline, an antitumor compound isolated from a sponge, induces G2/M cell cycle arrest and accumulation of polyubiquitinated p53. Biol. Pharm. Bull. 2004, 27, 699–701. [Google Scholar]
- Bickmeyer, U. Bromoageliferin and dibromoageliferin, secondary metabolites from the marine sponge Agelas conifera, inhibit voltage-operated, but not store-operated calcium entry in PC12 cells. Toxicon 2005, 45, 627–632. [Google Scholar]
- Liotta, L.A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986, 46, 1–7. [Google Scholar]
- Gross, H.; Kehraus, S.; Konig, G.M.; Woerheide, G.; Wright, A.D. New and biologically active imidazole alkaloids from two sponges of the genus Leucetta. J. Nat. Prod. 2002, 65, 1190–1193. [Google Scholar]
- Hassan, W.; Edrada, R.; Ebel, R.; Wray, V.; Berg, A.; van Soest, R.; Wiryowidagdo, S.; Proksch, P. New imidazole alkaloids from the Indonesian sponge Leucetta chagosensis. J. Nat. Prod. 2004, 67, 817–822. [Google Scholar]
- Tsukamoto, S.; Kawabata, T.; Kato, H.; Ohta, T.; Rotinsulu, H.; Mangindaan, R.E.; van Soest, R.W.; Ukai, K.; Kobayashi, H.; Namikoshi, M. Naamidines H and I, cytotoxic imidazole alkaloids from the Indonesian marine sponge Leucetta chagosensis. J. Nat. Prod. 2007, 70, 1658–1660. [Google Scholar]
- Copp, B.R.; Fairchild, C.R.; Cornell, L.; Casazza, A.M.; Robinson, S.; Ireland, C.M. Naamidine A is an antagonist of the epidermal growth factor receptor and an in vivo active antitumor. J. Med. Chem. 1998, 41, 3909–3911. [Google Scholar]
- James, R.D.; Jones, D.A.; Aalbersberg, W.; Ireland, C.M. Naamidine A intensifies the phosphotransferase activity of extracellular signal-regulated kinases causing A-431 cells to arrest in G1. Mol. Cancer Res. 2003, 2, 747–751. [Google Scholar]
- Aberle, N.; Catimel, J.; Nice, E.C.; Watson, K.G. Synthesis and biological evaluation of analogues of the anti-tumor alkaloid naamidine A. Bioorg. Med. Chem. Lett. 2007, 17, 3741–3744. [Google Scholar]
- Huigens, R.W., 3rd; Ma, L.; Gambino, C.; Moeller, P.D.; Basso, A.; Cavanagh, J.; Wozniak, D.J.; Melander, C. Control of bacterial biofilms with marine alkaloid derivatives. Mol. Biosyst. 2008, 4, 614–621. [Google Scholar]
- Stowe, S.D.; Tucker, A.T.; Thompson, R.; Piper, A.; Richards, J.J.; Rogers, S.A.; Mathies, L.D.; Melander, C.; Cavanagh, J. Evaluation of the toxicity of 2-aminoimidazole antibiofilm agents using both cellular and model organism systems. Drug Chem. Toxicol. 2012, 35, 310–315. [Google Scholar]
- Glowacka, I.E.; Balzarini, J.; Wroblewski, A.E. Design, synthesis, antiviral, and cytotoxic evaluation of novel phosphonylated 1,2,3-triazoles as acyclic nucleotide analogues. Nucleosides Nucleotides Nucleic Acids 2012, 31, 293–318. [Google Scholar]
- Balzarini, J.; Thomas, J.; Liekens, S.; Noppen, S.; Dehaen, W.; Romagnoli, R. 2-aminothiophene-3-carboxylic acid ester derivatives as novel highly selective cytostatic agents. Investig. New Drugs 2014, 32, 200–210. [Google Scholar]
- Ekwall, B. Screening of toxic compounds in mammalian cell cultures. Ann. N. Y. Acad. Sci. 1983, 407, 64–77. [Google Scholar]
- Ekwall, B.; Silano, A.; Paganuzzi-stammati, A.; Zucco, F. Toxicity Tests with Mammalian Cell Cultures. In Short-term Toxicity Tests for Non-genotoxic Effects; Bourdeau, P., Ed.; John Wiley & Sons Ltd: Hoboken, NJ, USA, 1990; pp. 75–99. [Google Scholar]
- Leung, M.C.; Williams, P.L.; Benedetto, A.; Au, C.; Helmcke, K.J.; Aschner, M.; Meyer, J.N. Caenorhabditis elegans: An emerging model in biomedical and environmental toxicology. Toxicol. Sci. 2008, 106, 5–28. [Google Scholar]
- Antoshechkin, I.; Sternberg, P.W. The versatile worm: Genetic and genomic resources for Caenorhabditis elegans research. Nat. Rev. Genet. 2007, 8, 518–532. [Google Scholar]
- Kaletta, T.; Hengartner, M.O. Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 2006, 5, 387–398. [Google Scholar]
- Glatt, V.; Kwong, F.N.; Park, K.; Parry, N.; Griffin, D.; Vrahas, M.; Evans, C.H.; Harris, M. Ability of recombinant human bone morphogenetic protein 2 to enhance bone healing in the presence of tobramycin: evaluation in a rat segmental defect model. J. Orthop. Trauma 2009, 23, 693–701. [Google Scholar]
- O’Callaghan, D.; Vergunst, A. Non-mammalian animal models to study infectious disease: Worms or fly fishing? Curr. Opin. Microbiol. 2010, 13, 79–85. [Google Scholar]
- Sample Availability: Samples of the compounds 1–40 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steenackers, H.; Dubey, A.; Robijns, S.; Ermolat'ev, D.; Delattin, N.; Dovgan, B.; Girandon, L.; Fröhlich, M.; De Brucker, K.; Cammue, B.P.A.; et al. Evaluation of the Toxicity of 5-Aryl-2-Aminoimidazole-Based Biofilm Inhibitors against Eukaryotic Cell Lines, Bone Cells and the Nematode Caenorhabditis elegans. Molecules 2014, 19, 16707-16723. https://doi.org/10.3390/molecules191016707
Steenackers H, Dubey A, Robijns S, Ermolat'ev D, Delattin N, Dovgan B, Girandon L, Fröhlich M, De Brucker K, Cammue BPA, et al. Evaluation of the Toxicity of 5-Aryl-2-Aminoimidazole-Based Biofilm Inhibitors against Eukaryotic Cell Lines, Bone Cells and the Nematode Caenorhabditis elegans. Molecules. 2014; 19(10):16707-16723. https://doi.org/10.3390/molecules191016707
Chicago/Turabian StyleSteenackers, Hans, Akanksha Dubey, Stijn Robijns, Denis Ermolat'ev, Nicolas Delattin, Barbara Dovgan, Lenart Girandon, Mirjam Fröhlich, Katrijn De Brucker, Bruno P. A. Cammue, and et al. 2014. "Evaluation of the Toxicity of 5-Aryl-2-Aminoimidazole-Based Biofilm Inhibitors against Eukaryotic Cell Lines, Bone Cells and the Nematode Caenorhabditis elegans" Molecules 19, no. 10: 16707-16723. https://doi.org/10.3390/molecules191016707
APA StyleSteenackers, H., Dubey, A., Robijns, S., Ermolat'ev, D., Delattin, N., Dovgan, B., Girandon, L., Fröhlich, M., De Brucker, K., Cammue, B. P. A., Thevissen, K., Balzarini, J., Van der Eycken, E. V., & Vanderleyden, J. (2014). Evaluation of the Toxicity of 5-Aryl-2-Aminoimidazole-Based Biofilm Inhibitors against Eukaryotic Cell Lines, Bone Cells and the Nematode Caenorhabditis elegans. Molecules, 19(10), 16707-16723. https://doi.org/10.3390/molecules191016707





