Optimization of Process Parameters of Extraction of Amentoflavone, Quercetin and Ginkgetin from Taxus chinensis Using Supercritical CO2 Plus Co-Solvent
Abstract
:1. Introduction

2. Results and Discussion
2.1. UPLC Chromatogram

2.2. Selection of Extraction Time
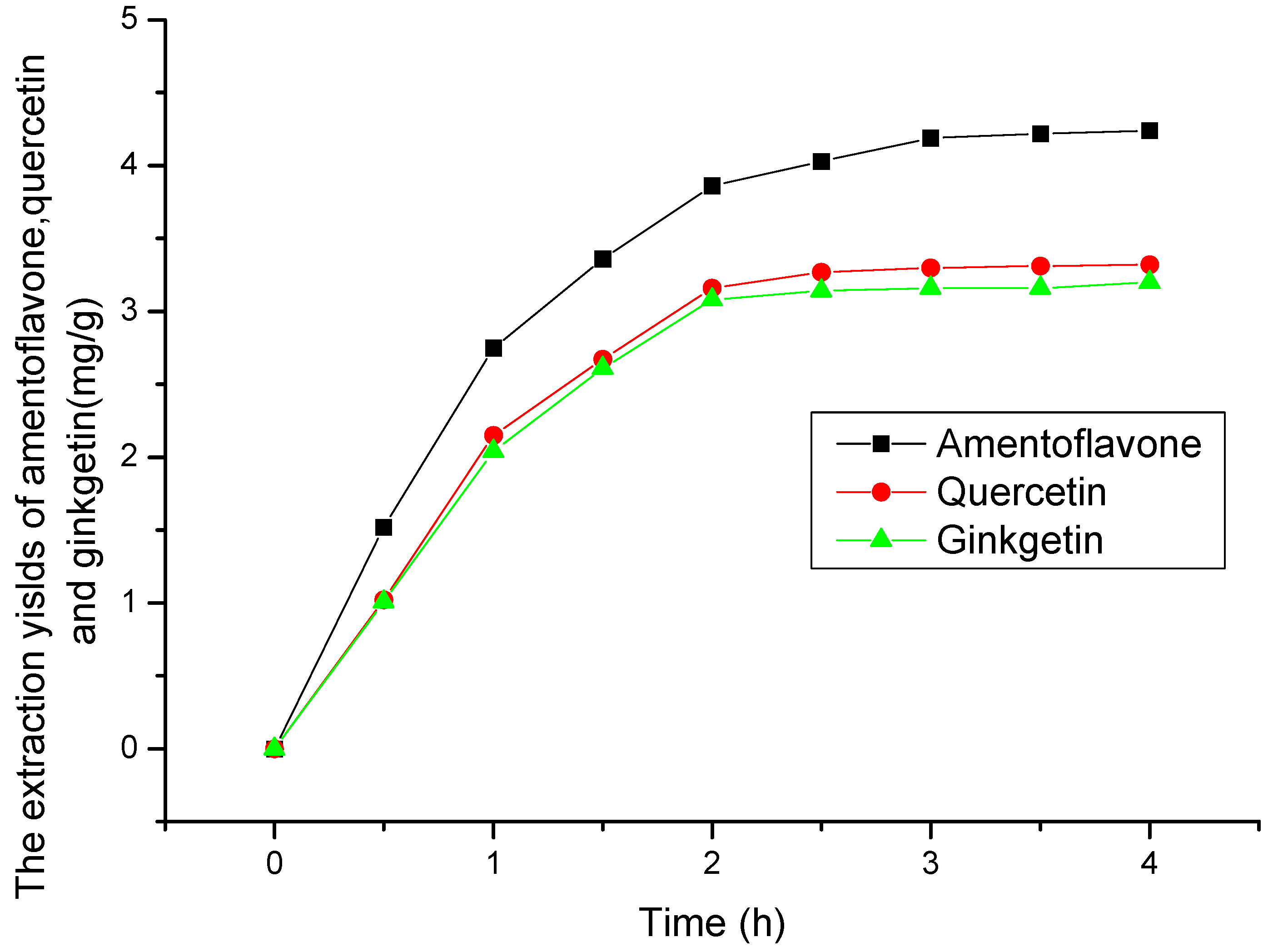
2.3. Model Fitting and Evaluation of the Factors
| Trail No. | X1 | X2 | X3 | X4 | Amentoflavone | Quercetin | Ginkgetin |
|---|---|---|---|---|---|---|---|
| Time (h) | Temperature (°C) | Pressure (bar) | Ethanol Concentration (ethanol/water, v/v, %) | Yield (mg/g) | Yield (mg/g) | Yield (mg/g) | |
| 1 | 2 | 45 | 20 | 100 | 3 | 1.52 | 1.19 |
| 2 | 2 | 45 | 20 | 60 | 3.01 | 1.64 | 1.11 |
| 3 | 2 | 45 | 20 | 80 | 4.35 | 3.53 | 3.27 |
| 4 | 2 | 45 | 30 | 80 | 4.37 | 3.56 | 3.21 |
| 5 | 2.5 | 40 | 25 | 70 | 3.92 | 3.28 | 2.1 |
| 6 | 1.5 | 50 | 15 | 90 | 3.29 | 2.32 | 2.19 |
| 7 | 1 | 45 | 20 | 80 | 3.28 | 2.91 | 2.74 |
| 8 | 2 | 35 | 20 | 80 | 3.95 | 3.21 | 2.57 |
| 9 | 1.5 | 50 | 25 | 70 | 3.83 | 2.84 | 2.61 |
| 10 | 1.5 | 40 | 25 | 90 | 3.62 | 2.82 | 2.18 |
| 11 | 2.5 | 50 | 25 | 70 | 4.22 | 3.31 | 2.97 |
| 12 | 2.5 | 40 | 25 | 90 | 4.02 | 3.13 | 2.55 |
| 13 | 2 | 45 | 20 | 80 | 4.35 | 3.53 | 3.27 |
| 14 | 2 | 55 | 20 | 80 | 4.35 | 3.57 | 3.18 |
| 15 | 2.5 | 40 | 15 | 70 | 3.55 | 3.02 | 1.89 |
| 16 | 1.5 | 40 | 25 | 70 | 3.51 | 2.87 | 2.16 |
| 17 | 1.5 | 40 | 15 | 70 | 2.93 | 2.89 | 1.96 |
| 18 | 1.5 | 40 | 15 | 90 | 3.23 | 2.02 | 2.22 |
| 19 | 1.5 | 50 | 25 | 90 | 3.84 | 3.01 | 2.54 |
| 20 | 2 | 45 | 20 | 80 | 4.35 | 3.54 | 3.26 |
| 21 | 2 | 45 | 20 | 80 | 4.34 | 3.53 | 3.27 |
| 22 | 2.5 | 50 | 15 | 70 | 3.62 | 3.22 | 2.22 |
| 23 | 2 | 45 | 10 | 80 | 3.24 | 2.87 | 2.57 |
| 24 | 2.5 | 50 | 15 | 90 | 3.83 | 2.68 | 2.65 |
| 25 | 2 | 45 | 20 | 80 | 4.33 | 3.54 | 3.27 |
| 26 | 1.5 | 50 | 15 | 70 | 3.05 | 3.05 | 2.11 |
| 27 | 2.5 | 50 | 25 | 90 | 4.12 | 3.34 | 2.96 |
| 28 | 3 | 45 | 20 | 80 | 4.39 | 3.68 | 3.35 |
| 29 | 2.5 | 40 | 15 | 90 | 3.67 | 2.21 | 2.56 |
| 30 | 2 | 45 | 20 | 80 | 4.33 | 3.54 | 3.27 |
| Source | Amentoflavone | Quercetin | Ginkgetin | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sum of Squares | F-value | p-value | R2 | Sum of Squares | F-value | p-value | R2 | Sum of Squares | F-value | p-value | R2 | |
| Model | 7.01 | 98.42 | <0.0001 | 0.9892 | 9.36 | 62.56 | <0.0001 | 0.9832 | 10.51 | 69.83 | <0.0001 | 0.9849 |
| X1 | 1.44 | 282.28 | <0.0001 | 0.64 | 59.6 | <0.0001 | 0.41 | 38.44 | <0.0001 | |||
| X2 | 0.19 | 37.87 | <0.0001 | 0.21 | 19.74 | 0.0005 | 0.62 | 57.42 | <0.0001 | |||
| X3 | 1.59 | 311.87 | <0.0001 | 0.87 | 81.42 | <0.0001 | 0.53 | 48.82 | <0.0001 | |||
| X4 | 0.039 | 7.71 | 0.0141 | 0.42 | 39.67 | <0.0001 | 0.16 | 15.34 | 0.0014 | |||
| X1X2 | 0.000506 | 0.1 | 0.7567 | 0.0053 | 0.49 | 0.4939 | 0.037 | 3.44 | 0.0832 | |||
| X1X3 | 0.03 | 5.85 | 0.0287 | 0.028 | 2.62 | 0.1260 | 0.0039 | 0.36 | 0.5558 | |||
| X1X4 | 0.0068 | 1.34 | 0.2654 | 6.3E-06 | 0.00058 | 0.9810 | 0.098 | 9.08 | 0.0087 | |||
| X2X3 | 0.018 | 3.45 | 0.0829 | 0.033 | 3.12 | 0.0979 | 0.15 | 13.96 | 0.0020 | |||
| X2X4 | 0.0045 | 0.9 | 0.3589 | 0.041 | 3.84 | 0.0690 | 0.059 | 5.47 | 0.0336 | |||
| X3X4 | 0.035 | 6.91 | 0.0190 | 0.54 | 50.89 | <0.0001 | 0.069 | 6.41 | 0.0230 | |||
| X12 | 0.51 | 100.27 | <0.0001 | 0.096 | 8.95 | 0.0091 | 0.16 | 14.87 | 0.0016 | |||
| X22 | 0.091 | 17.89 | 0.0007 | 0.034 | 3.2 | 0.0938 | 0.39 | 36.02 | <0.0001 | |||
| X32 | 0.57 | 111.6 | <0.0001 | 0.17 | 16.04 | 0.0011 | 0.36 | 33.79 | <0.0001 | |||
| X42 | 3.24 | 637.63 | <0.0001 | 6.53 | 610.66 | <0.0001 | 8.3 | 771.68 | <0.0001 | |||




| Extraction Condition | Amentoflavone | Quercetin | Ginkgetin | |||
|---|---|---|---|---|---|---|
| Dynamic extraction time, 2 h; temperature, 48 °C; pressure, 25 Mpa; Ethanol concentration,78%; flow-rate, 2 L/min CO2 | Yields (mg/g) (mean ± SD, n = 6) | |||||
| Experimental | 4.47 ± 0.06 | 3.65 ± 0.04 | 3.39 ± 0.02 | |||
| predicted | 4.47 | 3.69 | 3.40 | |||
| Purity (%) (mean ± SD, n = 6) | ||||||
| 9.41 ± 0.08 | 7.39 ± 0.05 | 6.02 ± 0.06 | ||||
| Soxhlet extractor; extraction solvent, methanol; extraction time, 7 h | Yields (mg/g) (mean ± SD, n = 6) | |||||
| 4.08 ± 0.03 | 2.96 ± 0.03 | 2.17 ± 0.02 | ||||
| Purity (%) (mean ± SD, n = 6) | ||||||
| 2.31 ± 0.02 | 1.83 ± 0.02 | 1.48 ± 0.02 | ||||
3. Experimental Section
3.1. Materials and Reagents
3.2. Supercritical Fluid Extraction
3.3. Soxhlet Extraction
3.4. UPLC Analysis
3.5. Experimental Design

| Factors | Coded Symbols | Levels | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | ||
| Extraction time (h) | X1 | 1 | 1.5 | 2 | 2.5 | 3 |
| Extraction temperature (°C) | X2 | 35 | 40 | 45 | 50 | 55 |
| Extraction pressure (MPa) | X3 | 10 | 15 | 20 | 25 | 30 |
| Ethanol concentration (ethanol/water, v/v, %) | X4 | 60 | 70 | 80 | 90 | 100 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rezende, T.P.; Correa, J.O.; Aarestrup, B.J.; Aarestrup, F.M.; de Sousa, O.V.; da Silva Filho, A.A. Protective effects of Baccharis dracunculifolia leaves extract against carbon tetrachloride- and acetaminophen-induced hepatotoxicity in experimental animals. Molecules 2014, 19, 9257–9272. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.Y.; Wang, X.X.; Gao, G.; Wang, L.; Li, Y.X.; Sun, C.J. Identification and quantification of free, conjugate and total phenolic compounds in leaves of 20 sweetpotato cultivars by HPLC-DAD and HPLC-ESI-MS/MS. Food Chem. 2013, 141, 2697–2706. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Zhu, M.X.; Han, X.J.; Sui, D.; Wang, Y.; Yang, Q. The metabolism of salidroside to its aglycone p-tyrosol in rats following the administration of salidroside. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Znati, M.; Jannet, B.; Cazaux, S.; Bouajila, J. Chemical composition, biological and cytotoxic activities of plant extracts and compounds isolated from Ferula lutea. Molecules 2014, 19, 2733–2747. [Google Scholar] [CrossRef] [PubMed]
- Ragab, F.A.; Yahya, T.; El-Naa, M.M.; Arafa, R.K. Design, synthesis and structure-activity relationship of novel semi-synthetic flavonoids as antiproliferative agents. Eur. J. Med. Chem. 2014, 82, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Anouar, E.; Shah, S.; Hassan, N.B.; El Moussaoui, N.; Ahmad, R.; Zulkefeli, M.; Weber, J. Antioxidant activity of hispidin oligomers from medicinal fungi: A DFT study. Molecules 2014, 19, 3489–3507. [Google Scholar] [CrossRef]
- Sun, L.; Simmerling, C.; Ojima, I. Recent advances in the study of the bioactive conformation of taxol. ChemMedChem 2009, 4, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.B.; Wu, Y.L.; Zhou, J.; Pan, Y.J. Structural characterisation of a water-soluble polysaccharide with high branches from the leaves of Taxus chinensis var. mairei. Food Chem. 2009, 113, 1020–1024. [Google Scholar] [CrossRef]
- Ruan, X.; Zhan, L.M.; Gao, X.X.; Yan, L.Y.; Zhang, H.; Zhu, Z.Y.; Wang, Q.; Jiang, D.A. Separation and purification of flavonoid from Taxus remainder extracts free of taxoids using polystyrene and polyamide resin. J. Sep. Sci. 2013, 36, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Lien, H.M.; Chiu, C.H.; Chen, C.C.; Chang, W.L.; Chyau, C.C.; Peng, R.Y. Comparison of the apoptotic effects of supercritical fluid extracts of Antrodia cinnamomea mycelia on hepatocellular carcinoma cells. Molecules 2014, 19, 9033–9050. [Google Scholar] [CrossRef] [PubMed]
- Saviano, A.M.; Francisco, F.L.; Lourenco, F.R. Rational development and validation of a new microbiological assay for linezolid and its measurement uncertainty. Talanta 2014, 127, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Mo, L.M.; Chen, F.; Lu, M.Q.; Dong, W.J.; Wang, Q.H.; Xu, F.; Gu, F.L. Optimized production of vanillin from green vanilla pods by enzyme-assisted extraction combined with pre-freezing and thawing. Molecules 2014, 19, 2181–2198. [Google Scholar] [CrossRef] [PubMed]
- Benelli, P.; Riehl, C.; Smania, A.; Smania, E.; Ferreira, S. Bioactive extracts of orange (Citrus sinensis L. Osbeck) pomace obtained by SFE and low pressure techniques: Mathematical modeling and extract composition. J. Supercrit. Fluids 2010, 55, 132–141. [Google Scholar] [CrossRef]
- Erkucuk, A.; Akgun, I.H.; Yesil-Celiktas, O. Supercritical CO2 extraction of glycosides from Stevia rebaudiana leaves: Identification and optimization. J. Supercrit. Fluids 2009, 51, 29–35. [Google Scholar] [CrossRef]
- Herrero, M.; Castro-Puyana, M.; Mendiola, J.A.; Ibanez, E. Compressed fluids for the extraction of bioactive compounds. TrAC Trends Anal. Chem. 2013, 43, 67–83. [Google Scholar] [CrossRef]
- Liu, X.; Yang, D.L.; Liu, J.J.; Xu, K.; Wu, G.H. Modeling of supercritical fluid extraction of flavonoids from Calycopteris floribunda leaves. Chem. Pap. 2014, 68, 316–323. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, X.; Yan, L.-Y.; Li, X.-X.; Liu, B.; Zhang, H.; Wang, Q. Optimization of Process Parameters of Extraction of Amentoflavone, Quercetin and Ginkgetin from Taxus chinensis Using Supercritical CO2 Plus Co-Solvent. Molecules 2014, 19, 17682-17696. https://doi.org/10.3390/molecules191117682
Ruan X, Yan L-Y, Li X-X, Liu B, Zhang H, Wang Q. Optimization of Process Parameters of Extraction of Amentoflavone, Quercetin and Ginkgetin from Taxus chinensis Using Supercritical CO2 Plus Co-Solvent. Molecules. 2014; 19(11):17682-17696. https://doi.org/10.3390/molecules191117682
Chicago/Turabian StyleRuan, Xiao, Liu-Ye Yan, Xian-Xian Li, Ben Liu, Huan Zhang, and Qiang Wang. 2014. "Optimization of Process Parameters of Extraction of Amentoflavone, Quercetin and Ginkgetin from Taxus chinensis Using Supercritical CO2 Plus Co-Solvent" Molecules 19, no. 11: 17682-17696. https://doi.org/10.3390/molecules191117682





