UV-Absorption—The Primary Process in Photocatalysis and Some Practical Consequences
Abstract
:1. Introduction
2. A Summary of Small Particle Optics (Where Diameter, d, Is Comparable with the Radiation Wavelength, λ)
2.1. Background Theory



2.2. Comparison of Theory and Experiment


2.3. The Major Problem Associated with the Calculation of Attenuation by Semi-Conductor Dispersions


| Base Crystal & Surface-Treatment | XRDLine Broadening | Equivalent Sphere Diameter from BET Area | SedimentationBrookhaven | Dynamic Light Scattering (DLS) or Laser Diffraction |
|---|---|---|---|---|
| 1. Ishihara ST-01 Anatase Untreated [37] | 7 | 5 | - | 2329 Beckman Coulter LS-230 Laser Diffraction |
| 2. Rutile-A silica-alumina [35] | 15 | 12 | 91 ± 10 | 133 (DLS) |
| 3: Rutile-A Stearate [35] | 15 | 23 | 53 ± 10 | 124 (DLS) |
| 4: Rutile-B Stearate [35] | 9 | 48 | 160 ± 19 | 201 (DLS) |
3. The Need to Consider UV Absorption when Interpreting Photocatalytic Results
3.1. Protection against Photodegradation of Organic Materials by Dispersed TiO2
3.1.1. Photodegradation of Polyethylene Films
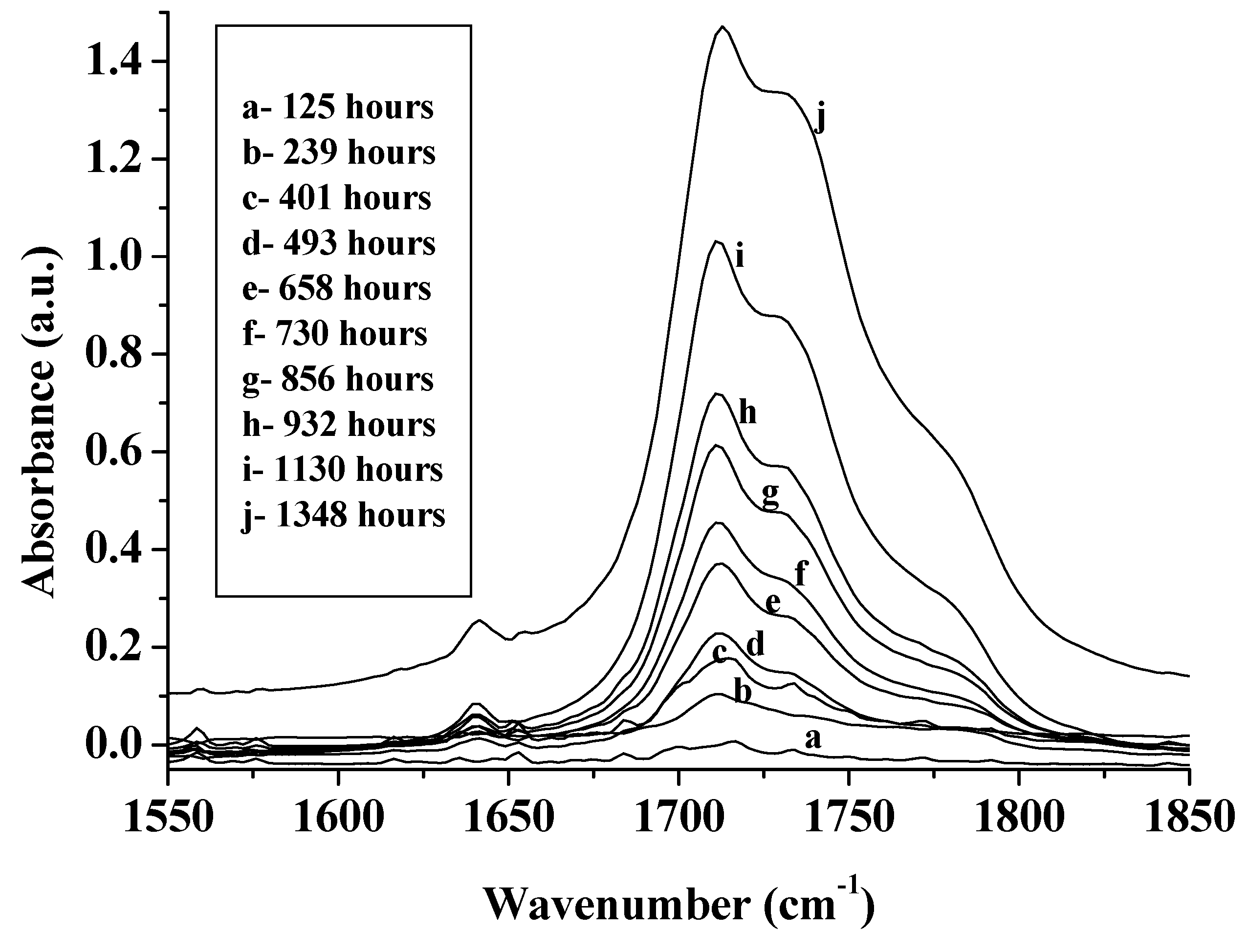


3.1.2. Photodegradation of Alkyd Paint Films

3.1.3. Disinfection by UV-C
| Conditions | Pre-Treated Wastewater | E. coli | ||
|---|---|---|---|---|
| Initial Number of Colony Forming Units (c.f.u.) | C.f.u after 60 min Treatment | Initial Number of Colony Forming Units (c.f.u.) | C.f.u after 60 min Treatment | |
| Lamp A | ~6 × 104 | Not detectable | 3 × 106 | Not detectable |
| Lamp A+TiO2 | 3 × 105 | 1.5 × 104 | 6 × 106 | 1 × 105 |
| Lamp B | 1 × 104 | 2 × 102 | ~8 × 106 | ~6.5 × 105 |
| Lamp B+TiO2 | 3 × 104 | 6 × 103 | ~8 × 106 | 1.5 × 101 |
3.2. Reduced Photocatalytic Activity of Other Solution Species because of UV Absorption by Nano-Particulate TiO2

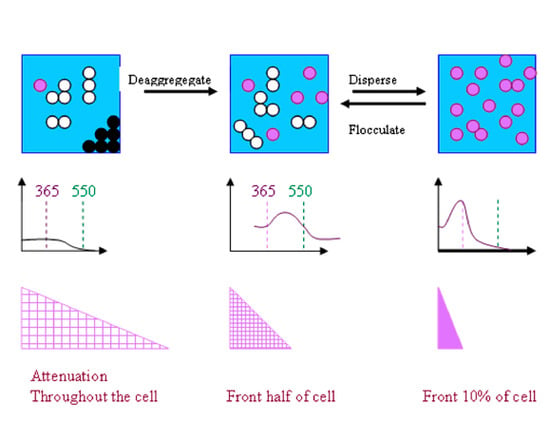
3.3. Changes in UV Absorption Caused by Agglomeration or Flocculation Alter the Measured Photocatalytic Activity of Nano-Particles
3.3.1. Predicted Change in Photocatalytic Activity Resulting from an Increase in UV Absorption

3.3.2. Effect of Milling on Photocatalytic Activity for Propan-2-ol Oxidation

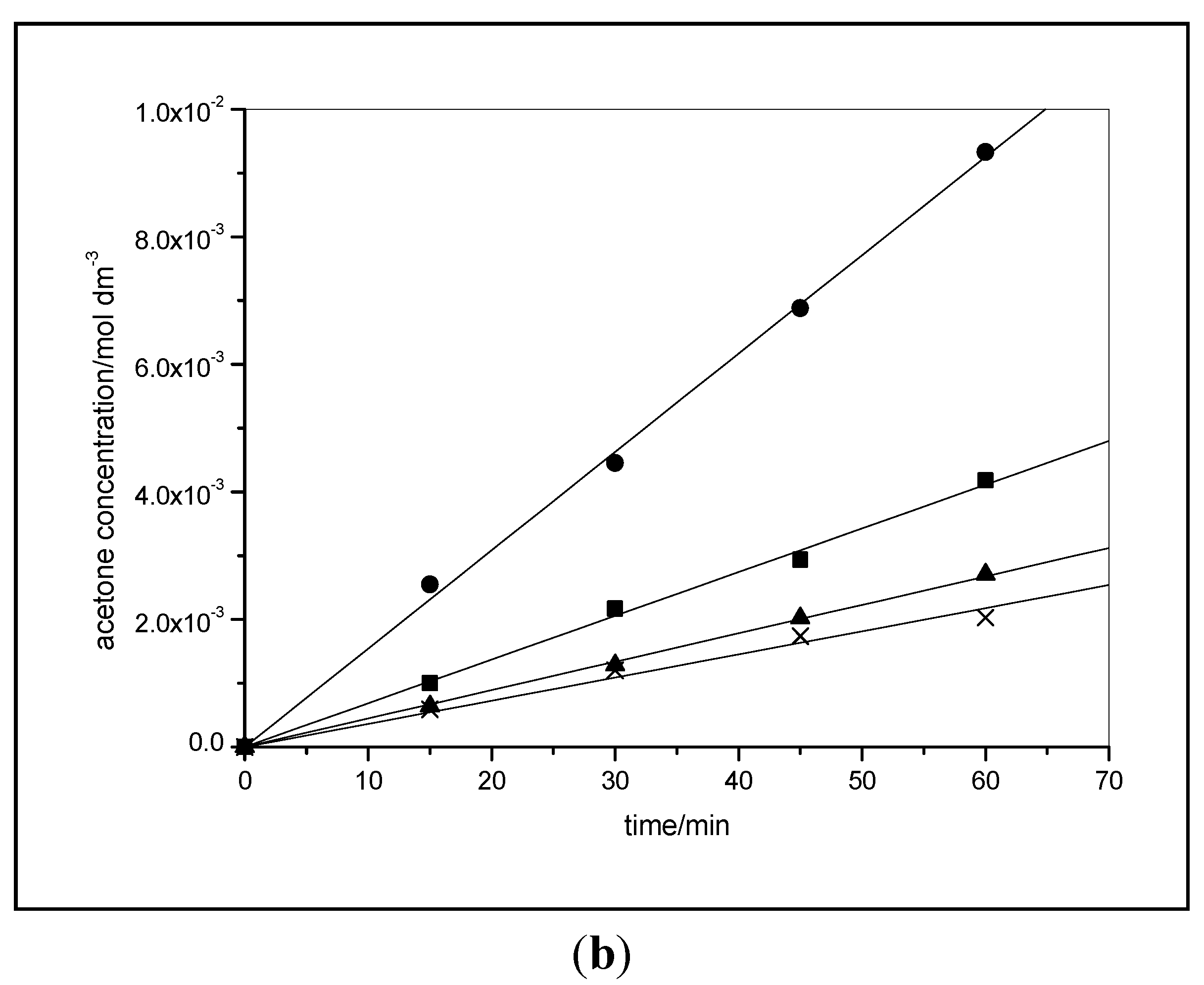
3.3.3. Effect of Milling on Photocatalytic Degradation of Salicylic Acid

3.3.4. Effect of Milling on Photocatalytic Degradation of Dichloroacetic Acid

4. Conclusions and Implications
Acknowledgments
Conflicts of Interest
References
- Matsunaga, T.; Okochi, M. TiO2-Mediated Photochemical Disinfection of Escherichia coli Using Optical Fibers. Environ. Sci. Technol. 1995, 29, 501–505. [Google Scholar]
- Maillard, C.; Guillard, C.; Pichat, P. Comparative effects of the TiO2-UV, H2O2-UV H2O2-Fe2+ systems on The disappearance Rate of benzamide and 4-hydroxybenzamide in water. Chemosphere 1992, 24, 1085–1094. [Google Scholar]
- Mills, A.; Belghazi, A.; Davies, R.H.; Morris, S.A. Kinetic study of the bleaching of Rhodamine 6G photosensitized by titanium dioxide. J. Photochem. Photobiol. A 1994, 79, 131–139. [Google Scholar]
- Fateh, R.; Dillert, R.; Bahnemann, D. Self-cleaning properties, mechanical stability, and adhesion strength of transparent photocatalytic TiO2-ZnO coatings on polycarbonate. ACS Appl. Mater. Interfaces 2014, 6, 2269–2277. [Google Scholar]
- Hughes, W. Phodegradation of paint films containing TiO2 pigments. In Xth FATIPEC Congress 1970; Verlag Chemie GmbH: Weinheim/Bergst, Germany, 1970; pp. 67–82. [Google Scholar]
- Allen, N.S.; McKellar, J.F.; Phillips, G.O.; Chapman, C.B. The TiO2 photosensitized degradation of nylon 6, 6: Stabilizing action of manganese ions. J. Polym. Sci. C Polym. Lett. 1974, 12, 723–727. [Google Scholar]
- Turchi, C.S.; Ollis, D.F. Photocatalytic degradation of organic-water contaminants—Mechanisms involving hydroxyl radical attack. J. Catal. 1990, 122, 178–192. [Google Scholar]
- Egerton, T.A.; King, C.J. The Influence of Light Intensity on Photoactivity in TiO2 pigmented systems. J. Oil Colour Chem. Assoc. 1979, 62, 386–391. [Google Scholar]
- Bard, A.J. Heterogeneous photocatalytic decomposition of saturated carboxylic acids on TiO2 powder decarboxylative route to alkanes. J. Am. Chem. Soc. 1978, 100, 5985–5992. [Google Scholar]
- Mills, A.; LeHunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A 1997, 108, 1–35. [Google Scholar]
- Glasstone, S. Textbook of Physical Chemistry, 2nd ed.; Macmillan: London, UK, 1962; p. 1155. [Google Scholar]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar]
- Vinodgopal, K.; Wynkoop, D.E.; Kamat, P.V. Environmental photochemistry on semiconductor surfaces: Photosensitized degradation of a textile azo dye, acid orange 7, on TiO2 particles using visible light. Environ. Sci. Technol. 1996, 30, 1660–1666. [Google Scholar]
- Chen, Y.S.; Crittenden, J.C.; Hackney, S.; Sutter, L.; Hand, D.W. Preparation of a novel TiO2-based p-n junction nanotube photocatalyst. Environ. Sci. Technol. 2005, 39, 1201–1208. [Google Scholar]
- Morawski, A.W.; Grzechulska, J.; Kalucki, K. A new method for the preparation of potassium-pillared layered titanate applied in photocatalysis. J. Phys. Chem. Solids 1996, 57, 1011–1017. [Google Scholar]
- Pelizzetti, E.; Carlin, V.; Minero, C.; Pramauro, E.; Vincenti, M. Degradation pathways of atrazine under solar light and in the presence of TiO2 colloidal particles. Sci. Total Environ. 1992, 123, 161–169. [Google Scholar]
- Wang, L.; Egerton, T.A. The influence of chromium on photocatalysis of propan-2-ol and octadecanoic acid oxidation by rutile TiO2. J. Photochem. Photobiol. A 2013, 252, 211–215. [Google Scholar]
- Litter, M.I. Heterogeneous photocatalysis-transition metal ions in photocatalytic systems. Appl. Catal. B Environ. 1999, 23, 89–114. [Google Scholar]
- Egerton, T. Does photoelectrocatalysis by TiO2 work? J. Chem. Technol. Biotechnol. 2011, 86, 1024–1031. [Google Scholar]
- Rayleigh, L. On the light from the sky, its polarization and colour. Philos. Mag. 1871, 41, 107–120, 274–279. [Google Scholar]
- Mie, G. Articles on the optical characteristics of turbid tubes, especially colloidal metal solutions. Ann. Phys. 1908, 25, 377–445. [Google Scholar]
- Vos, K.; Krusemeyer, H.J. Reflectance and electroreflectance of TiO2 single crystals. J. Phys. C Solid State Phys. 1977, 10, 3893. [Google Scholar]
- Egerton, T.A.; Tooley, I.R. UV absorption and scattering properties of inorganic-based sunscreens. Int. J. Cosmet. Sci. 2012, 34, 117–122. [Google Scholar]
- Robb, J.L.; Simpson, L.A.; Tunstall, D.F. Titanium dioxide and UV radiation. Drug Cosmet. Ind. 1994, 154, 32–39. [Google Scholar]
- Ghosh, A.K.; Maruska, H.P. Photoelectrolysis of water in sunlight on sensitized semiconductor electrodes. J. Electrochem. Soc. 1977, 124, 1516–1522. [Google Scholar]
- Mollers, F.; Tolle, H.J.; Memming, R. On the origin of photocatalytic deposition of noble metals on TiO2. J. Electrochem. Soc. 1974, 121, 1160–1167. [Google Scholar]
- Martin, C.A.; Baltanas, M.A.; Cassano, A.E. Photocatalytic reactors II. Quantum efficiencies allowing for scattering effects. An experimental approximation. J. Photochem. Photobiol. A 1996, 94, 173–189. [Google Scholar]
- Romero, R.L.; Alfano, O.M.; Cassano, A.E. Cylindrical Photocatalytic Reactors. Radiation absorption and scattering effects produced by suspended fine particles in an annular space. Ind. Eng. Chem. Res. 1997, 36, 3094–3109. [Google Scholar]
- Satuf, M.A.; Brandi, R.J.; Cassano, A.E.; Orlando, M.A. Experimental method to evaluate the optical properties of aqueous titanium dioxide suspensions. Ind. Eng. Chem. Res. 2005, 44, 6643–6649. [Google Scholar]
- Marugan, J.; van Grieken, R.; Pablos, C.; Satuf, M.L.; Cassano, A.E. Modelling of bench-scale photocatalytic reactor for water disinfection from laboratory-scale data. Chem. Eng. J. 2013, 2245, 39–45. [Google Scholar]
- Toepfer, B.; Gora, A.; Li Puma, G. Photocatalytic oxidation of multicomponent solutions of herbicides Reaction kinetics with explicit photon absorption effects. Appl. Catal. B Environ. 2006, 68, 171–180. [Google Scholar]
- Angel Mueses, M.; Machuca-Martinez, F.; Li Puma, G. Effective quantum yield and reaction rate model for evaluation of photocatalytic degradation of water contaminants in heterogeneous pilot-scale solar photoreactors. Chem. Eng. J. 2013, 215–216, 937–947. [Google Scholar]
- Tunstall, D.F.; Hird, M.J. Effect of particle crowding on scattering power of TiO2 pigments. J. Paint Technol. 1974, 46, 33–40. [Google Scholar]
- Martin, C.A.; Baltanas, M.A.; Cassano, A.E. Photocatalytic reactors I. Optical behaviour of titanium oxide particulate suspensions. J. Photochem. Photobiol. A 1993, 76, 199–208. [Google Scholar]
- Egerton, T.A.; Tooley, I.R. Physical characterization of titanium dioxide nanoparticles. Int. J. Cosmet. Sci. 2014, 36, 195–206. [Google Scholar]
- Egerton, T.A.; Tooley, I.R. Effect of changes in TiO2 dispersion on its measured photocatalytic activity. J. Phys. Chem. B 2004, 108, 5066–5072. [Google Scholar]
- Ridley, M.K.; Hackley, V.A.; Machesky, M.L. Characterization and Surface Reactivity of Nanocrystalline Anatase in Aqueous Solutions. Langmuir 2006, 22, 10972–10982. [Google Scholar]
- French, R.A.; Jacobson, A.R.; Kim, B.; Isley, S.L.; Penn, R.L.; Baveye, P.C. Influence of Ionic Strength, pH, and Cation Valence on Aggregation Kinetics of Titanium Dioxide Nanoparticles. Environ. Sci. Technol. 2009, 43, 1354–1359. [Google Scholar]
- Lee, B.C.; Kim, K.T.; Cho, J.G.; Lee, J.W.; Ryu, T.K.; Yoon, J.H.; Lee, S.H.; Duong, C.N.; Eom, I.C.; Ki, P.J.; et al. Oxidative stress in juvenile common carp (Cyprinus carpio) exposed to TiO2 nanoparticles. Mol. Cell. Toxicol. 2012, 8, 357–366. [Google Scholar]
- Egerton, T.A.; Harrison, R.W.; Hill, S.E.; John, A.; Mattinson, J.A.; Purnama, H. Effects of particle dispersion on the measurement of semi-conductor photocatalytic activity. J. Photochem. Photobiol. A 2010, 216, 268–274. [Google Scholar]
- Fernando, S.S.; Christensen, P.A.; Egerton, T.A.; White, J.R. Carbon dioxide evolution and carbonyl group development during photodegradation of polyethylene and polypropylene. Polym. Degrad. Stab. 2007, 92, 2163–2172. [Google Scholar]
- Egerton, T.A. The modification of fine powders by inorganic coatings. Kona 1998, 16, 46–59. [Google Scholar]
- Egerton, T.A. Titanium Compounds, organic. In Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed.; John Wiley & Sons Inc.: New York, NY, USA, 1997; Volume 24, pp. 225–274. [Google Scholar]
- Egerton, T.A.; Tooley, I.R. The surface characterization of coated titanium dioxide by FTIR spectroscopy of adsorbed nitrogen. J. Mater. Chem. 2002, 12, 1111–1117. [Google Scholar]
- Worsley, D.A.; Searle, J.R. Photoactivity test for TiO2 pigment photocatalysed polymer degradation. Mater. Sci. Technol. Lond. 2002, 18, 681–684. [Google Scholar]
- Jin, C. FTIR Studies of TiO2-pigmented Polymer Photodegradation. Ph.D. Thesis, University of Newcastle, Newcastle, UK, 1974. [Google Scholar]
- Christensen, P.A.; Dilks, A.; Egerton, T.A.; Lawson, E.J.; Temperley, J. Photocatalytic oxidation of alkyd paint films measured by FTIR analysis of UV generated carbon dioxide. J. Mater. Sci. 2002, 37, 4901–4909. [Google Scholar]
- Dillert, R.; Siemon, U.; Bahnemann, D. Photocatalytic disinfection of municipal wastewater. Chem. Eng. Technol. 1998, 21, 356–360. [Google Scholar]
- Gupta, V.K.; Jain, R.; Agarwal, S.; Shrivastava, M. Kinetics of photocatalytic degradation of tropaeoline 000 using UV/TiO2 in a UV reactor. Colloid Surf. A 2011, 378, 22–26. [Google Scholar]
- Wu, C.H. Effects of operational parameters on the decolourization of C.I. reactive red 198 in UV/TiO2 systems. Dyes Pigment. 2008, 77, 31–38. [Google Scholar]
- Sauer, T.; Neto, C.; Jose, H.J.; Moreira, R.F.P.M. Kinetics of photocatalytic degradation of reactive dyes in a TiO2 slurry reactor. J. Photochem. Photobiol. A 2002, 149, 147–154. [Google Scholar]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic Degradation of azo acid red 14 in water: Investigation of the effect of operational parameters. J. Photochem. Photobiol. A 2003, 157, 111–116. [Google Scholar]
- Egerton, T.A.; Purnama, H. Does hydrogen peroxide really accelerate TiO2 UV-C photocatalyzed decolouration of azo dyes such as Reactive Orange 16? Dyes Pigment. 2014, 101, 280–285. [Google Scholar]
- Baxendale, J.H.; Wilson, J.A. Photolysis of hydrogen peroxide at high light intensities. Trans. Faraday Soc. 1957, 53, 344–356. [Google Scholar]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Photolysis of Chloroform and other organic molecules in aqueous TiO2 suspensions. Environ. Sci. Technol. 1991, 25, 494–500. [Google Scholar]
- Blake, D.M.; Webb, J.; Turchi, C.; Magrine, K. Kinetic and Mechanistic Overview of TiO2. Sol. Energy Mater. 1991, 24, 584–593. [Google Scholar]
- Okamoto, K.; Yamamoto, Y.; Tanaka, H.; Itaya, A. Kinetics of heterogeneous photocatalytic decomposition of phenol over anatase powder. Bull. Chem. Soc. Jpn. 1985, 58, 2023–2028. [Google Scholar]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egerton, T.A. UV-Absorption—The Primary Process in Photocatalysis and Some Practical Consequences. Molecules 2014, 19, 18192-18214. https://doi.org/10.3390/molecules191118192
Egerton TA. UV-Absorption—The Primary Process in Photocatalysis and Some Practical Consequences. Molecules. 2014; 19(11):18192-18214. https://doi.org/10.3390/molecules191118192
Chicago/Turabian StyleEgerton, Terry A. 2014. "UV-Absorption—The Primary Process in Photocatalysis and Some Practical Consequences" Molecules 19, no. 11: 18192-18214. https://doi.org/10.3390/molecules191118192
APA StyleEgerton, T. A. (2014). UV-Absorption—The Primary Process in Photocatalysis and Some Practical Consequences. Molecules, 19(11), 18192-18214. https://doi.org/10.3390/molecules191118192