Chitin and Chitosan Nanofibers: Preparation and Chemical Modifications
Abstract
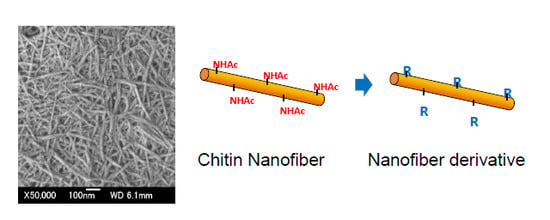
:1. Introduction

2. Preparation of Chitin and Chitosan Nanofibers
2.1. Preparation of Chitin Nanofibers
2.1.1. Nanofibers from Crab Shell [12]


2.1.2. Nanofibers from Prawn Shell [17]
2.1.3. Nanofibers from Mushroom [20]
2.1.4. Nanofibers from Squid Pen e-Chitin [24,25]
2.1.5. Nanofibers from Commercial Chitin Powder [26,27,28]
2.2. Preparation of Chitosan Nanofibers [29]

3. Chemical Modifications of Chitin Nanofibers
3.1. Acetylation of Chitin Nanofibers [36]

3.2. Preparation of Partially Deacetylated Chitin Nanowhiskers [41]

3.3. Surface Maleylation, Phthaloylation, and Naphthaloylation of Chitin Nanofibers [42,43]

3.4. Preparation of Surface N-Halamine Chitin Nanofibers [46]

3.5. Chitin Nanocrystals Prepared by TEMPO-Mediated Oxidation [50]

3.6. Graft Polymerization of Acrylic Acid onto Chitin Nanofiber [51]

4. Conclusions
Conflicts of Interest
References
- Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yin, Y.; Kim, F.; Yan, H. One-dimensional nanostructures: Synthesis, characterization, and applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Yano, H.; Sugiyama, J.; Nakagaito, A.N.; Nogi, M.; Matsuura, T.; Hikita, M.; Handa, K. Optically transparent composites reinforced with networks of bacterial nanofibers. Adv. Mater. 2005, 17, 153–155. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Iwamoto, S.; Yano, H. Bacterial cellulose: The ultimate nano-scalar cellulose morphology for the production of high-strength composites. Appl. Phys. A Mater. Sci. Process. 2005, 80, 93–97. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jeong, L.; Kang, Y.O.; Lee, S.O.; Park, W.H. Electrospinning of polysachharides for regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1020–1032. [Google Scholar] [CrossRef]
- Sill, T.J.; Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, N.K.; Dufresne, A. Crab shells chitin whiskers reinforced natural rubber nanocomposites. 1. Processing and swelling behavior. Biomacromolecules 2003, 4, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Raabe, D.; Sachs, C.; Romano, P. The crustacean exoskeleton as an example of a structurally and mechanically graded biological nanocomposite material. Acta Mater. 2005, 53, 4281–4292. [Google Scholar] [CrossRef]
- Ifuku, S.; Saimoto, H. Chitin nanofibers: Preparations, modifications, and applications. Nanoscale 2012, 4, 3308–3318. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Ifuku, S.; Osaki, T.; Okamoto, Y.; Minami, S. Preparation and biomedical applications of chitin and chitosan nanofibers. J. Biomed. Nanotechnol. 2014, 10, 2891–2920. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of chitin nanofibers with a uniform width as a-chitin from crab shells. Biomacromolecules 2009, 10, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Shimahara, K.; Takiguchi, Y. Preparation of crustacean chitin. In Methods in Enzymology; Wood, W.A., Kellogg, S.T., Eds.; Academic Press: San Diego, CA, USA, 1998; Volume 6, pp. 417–423. [Google Scholar]
- BeMiller, J.N.; Whistler, R.L. Alkaline degradation of amino sugars. J. Org. Chem. 1962, 27, 1161–1164. [Google Scholar] [CrossRef]
- Abe, K.; Iwamoto, S.; Yano, H. Obtaining cellulose nanofibers with a uniform width of 15 nm from wood. Biomacromolecules 2007, 8, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, S.; Nakagaito, A.N.; Yano, H.; Nogi, M. Optically transparent composites reinforced with plant fiber-based nanofibers. Appl. Phys. A Mater. Sci. Process. 2005, 81, 1109–1112. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Simple preparation method of chitin nanofibers with a uniform width of 10–20 nm from prawn shell under neutral conditions. Carbohydr. Polym. 2011, 84, 762–764. [Google Scholar] [CrossRef]
- Chen, P.; Lin, A.Y.; Mckittrick, J.; Meyers, M.A. Structure and mechanical properties of crab exoskeletons. Acta Biomater. 2008, 4, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Yano, I. Structure and production of exoskeletons of crabs and prawns. Kagaku Seibutsu 1977, 15, 328–336. [Google Scholar] [CrossRef]
- Ifuku, S.; Nomura, R.; Morimoto, M.; Saimoto, H. Preparation of chitin nanofibers from mushrooms. Materials 2011, 4, 1417–1425. [Google Scholar] [CrossRef]
- Michalenko, G.O.; Hohl, H.R.; Rast, D. Chemistry and architecture of the mycelial wall of Agaricus bisporus. J. Gen. Microbiol. 1976, 92, 251–262. [Google Scholar] [CrossRef]
- Zivanovic, S.; Buescher, R.; Kim, S.K. Mushroom texture, cell wall composition, color, and ultrastructure as affected by pH and temperature. J. Food Sci. 2003, 68, 1860–1865. [Google Scholar] [CrossRef]
- Ivshina, T.N.; Artamonova, S.D.; Ivshin, V.P.; Sharnina, F.F. Isolation of the chitin-glucan complex from the fruiting bodies of mycothallus. Appl. Biochem. Microbiol. 2009, 45, 313–318. [Google Scholar] [CrossRef]
- Fan, Y.; Saito, T.; Isogai, A. Preparation of chitin nanofibers from squid pen β-chitin by simple mechanical treatment under acid conditions. Biomacromolecules 2008, 9, 1919–1923. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.K.; Izawa, H.; Morimoto, M.; Saimoto, H.; Ifuku, S. Simple preparation of chitin nanofibers from dry squid pen β-chitin powder by the star burst system. J. Chitin Chitosan Sci. 2013, 1, 186–191. [Google Scholar] [CrossRef]
- Ifuku, S.; Shervani, Z.; Saimoto, H. Preparation of chitin nanofibers nd their composites. In Biopolymer Nanocomposite; Dufresne, A., Thomas, S., Pothan, L.A., Eds.; Wiley: Hoboken, NJ, USA, 2013; Volume 2, pp. 11–32. [Google Scholar]
- Ifuku, S.; Yamada, K.; Morimoto, M.; Saimoto, H. Nanofibrillation of dry chitin powder by Star Burst system. J. Nanomater. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Dutta, A.K.; Yamada, K.; Izawa, H.; Morimoto, M.; Saimoto, H.; Ifuku, S. Preparation of chitin nanofibers from dry chitin powder by Star Burst system: Dependence on number of passes. J. Chitin Chitosan Sci. 2013, 1, 59–64. [Google Scholar] [CrossRef]
- Dutta, A.K.; Kawamoto, N.; Sugino, G.; Izawa, H.; Morimoto, M.; Saimoto, H.; Ifuku, S. Simple preparation of chitosan nanofibers from dry chitosan powder by the Star Burst system. Carbohydr. Polym. 2013, 97, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A. Chitin nanostructures in living organisms. In Chitin Formation and Diagenesis; Gupta, S.N., Ed.; Springer: Dordrecht, The Netherlands, 2011; Volume 34, pp. 1–34. [Google Scholar]
- Muzzarelli, R.A.A.; Boudrant, J.; Meyer, D.; Manno, N.; DeMarchis, M.; Paoletti, M.G. Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: A tribute to Henri Braconnot, precursor of the carbohydrate polymers science on the chitin bicentennial. Carbohydr. Polym. 2012, 87, 995–1012. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Nanochitins and nanochitosans, paving the way to eco-friendly and energy-saving exploitation of marine resources. In Polymer Science: A Comprehensive Reference; Maty-jaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 10, pp. 153–164. [Google Scholar]
- Peter, M.G. Applications and environmental aspects of chitin and chitosan. J. Macromol. Sci. A Pure Appl. Chem. 1995, 32, 629–640. [Google Scholar] [CrossRef]
- Rao, S.B.; Sharma, C.P. Use of chitosan as a biomaterial: Studies on its safety and hemostatic potential. J. Biomed. Mater. Res. 1997, 34, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Lee, S.W.; Lim, J.N.; You, Y.; Lee, T.S.; Kang, P.H.; Park, W.H. Chitinand chitosan nanofibers: Electrospinning of chitin and deacetylation of chitinnanofibers. Polymer 2004, 45, 7137–7142. [Google Scholar] [CrossRef]
- Ifuku, S.; Morooka, S.; Morimoto, M.; Saimoto, H. Acetylation of chitin nanofibers and their transparent nanocomposite films. Biomacromolecules 2010, 11, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K. Controlled functionalization of the polysaccharide chitin. Prog. Polym. Sci. 2001, 9, 1921–1971. [Google Scholar] [CrossRef]
- Nogi, M.; Abe, K.; Handa, K.; Nakatsubo, F.; Ifuku, S.; Yano, H. Property enhancement of optically transparent bionanofiber composites by acetylation. Appl. Phys. Lett. 2006, 89, 233123. [Google Scholar] [CrossRef] [Green Version]
- Ifuku, S.; Nogi, M.; Abe, K.; Handa, K.; Nakatsubo, F.; Yano, H. Surface modification of bacterial cellulose nanofibers for property enhancement of optically transparent composites: Dependence on acetyl-group DS. Biomacromolecules 2007, 8, 1973–1978. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Nishiyama, Y.; Kuga, S. Surface acetylation of bacterial cellulose. Cellulose 2002, 9, 361–367. [Google Scholar] [CrossRef]
- Fan, Y.; Saito, T.; Isogai, A. Individual chitin nano-whiskers prepared from partially deacetylated α-chitin by fibril surface cationization. Carbohydr. Polym. 2010, 79, 1046–1051. [Google Scholar] [CrossRef]
- Ifuku, S.; Suzuki, N.; Izawa, H.; Morimoto, M.; Saimoto, H. Surface phthaloylation of chitin nanofiber in aqueous media to improve dispersibility in aromatic solvents and give thermo-responsive and ultraviolet protection properties. RSC Adv. 2014, 4, 19246–19250. [Google Scholar] [CrossRef]
- Ifuku, S.; Suzuki, N.; Izawa, H.; Morimoto, M.; Saimoto, H. Surface maleylation and naphthaloylation of chitin nanofibers for property enhancement. React. Funct. Polym. 2014, in press. [Google Scholar]
- Kurita, K.; Ikeda, H.; Yoshida, Y.; Shimojoh, M.; Harata, M. Chemoselective protection of the amino groups of chitosan by controlled phthaloylation: Facile preparation of a precursor useful for chemical modifications. Biomacromolecules 2002, 3, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Miwa, T.; Morimoto, M.; Saimoto, H. Preparation of highly chemoselective N-phthaloyl chitosan in aqueous media. Green Chem. 2011, 13, 1499–1502. [Google Scholar]
- Dutta, A.K.; Egusa, M.; Kaminaka, H.; Izawa, H.; Morimoto, M.; Saimoto, H.; Ifuku, S. Facile preparation of surface N-halamine chitin nanofiber to endow antibacterial and antifungal activities. Carbohydr. Polym. 2015, 115, 342–347. [Google Scholar] [CrossRef]
- Kocer, H.B.; Cerkez, I.; Worley, S.D.; Broughton, R.M.; Huang, T.S. Poly-meric antimicrobial N-halamine epoxides. ACS Appl. Mater. Interfaces 2011, 3, 2845–2850. [Google Scholar] [CrossRef] [PubMed]
- Worley, S.D.; Li, F.; Wu, R.; Kim, J.; Wei, C.; Williams, J.F.; Owens, J.R.; Wander, J.D.; Bargmeyer, A.M.; Shirtiliff, M.E. A novel N-halamine monomer for preparing biocidal polyurethane coatings. Surf. Coat. Int. B 2003, 86, 273–277. [Google Scholar] [CrossRef]
- Worley, S.D.; Williams, D.E. Halamine water disinfectants. Crit. Rev. Environ. Sci. Technol. 1988, 18, 133–175. [Google Scholar]
- Fan, Y.; Saito, T.; Isogai, A. Chitin nanocrystals prepared by TEMPO-mediated oxidation of α-chitin. Biomacromolecules 2008, 9, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Iwasaki, M.; Morimoto, M.; Saimoto, H. Graft polymerization of acrylic acid onto chitin nanofiber to improve dispersibility in basic water. Carbohydr. Polym. 2012, 90, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Loría-Bastarrachea, M.I.; Carrillo-Escalante, H.J.; Aguilar-Vega, M.J. Grafting of poly(acrylic acid) onto cellulosic microfibers and continuous cellulose filaments and characterization. J. Appl. Polym. Sci. 2002, 10, 386–393. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Pourjavadi, A.; Hosseinzadeh, H.; Zohuriaan, M.J. Modified chitosan 4. superabsorbent hydrogels from poly(acrylic acid-coacrylamide) grafted chitosan with salt- and pH-responsiveness properties. Eur. Polym. J. 2004, 40, 1399–1407. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Y. Preparation and characterization of graft copolymerization of ethyl acrylate onto hydroxypropyl methylcellulose in aqueous medium. Cellulose 2006, 13, 191–200. [Google Scholar] [CrossRef]
- Abe, K.; Ifuku, S.; Kawata, M.; Yano, H. Preparation of tough hydrogels based on β-chitin nanofibers via NaOH treatment. Cellulose 2014, 21, 535–540. [Google Scholar] [CrossRef]
- Torres-Rendon, J.G.; Schacher, F.H.; Ifuku, S.; Walther, A. Mechanical performance of macrofibers of cellulose and chitin, nanofibrils aligned by wet-stretching: A critical comparison. Biomacromolecules 2014, 15, 2709–2717. [Google Scholar] [PubMed]
- Das, P.; Heuser, T.; Wolf, A.; Zhu, B.; Demco, D.E.; Ifuku, S.; Walther, A. Tough and catalytically active hybrid biofibers wet-spun from nanochitin hydrogels. Biomacromolecules 2012, 13, 4205–4212. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Morooka, S.; Nakagaito, A.N.; Morimoto, M.; Saimoto, H. Preparation and characterization of optically transparent chitin nanofiber/(meth)acrylic resin composites. Green Chem. 2011, 13, 1708–1711. [Google Scholar] [CrossRef]
- Ifuku, S.; Ikuta, A.; Hosomi, T.; Kanaya, S.; Shervani, Z.; Morimoto, M.; Saimoto, H. Preparation of polysilsesquioxane-urethaneacrylate copolymer film reinforced with chitin nanofibers. Carbohydr. Polym. 2012, 89, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Nakata, K.; Ikuta, A.; Oba, T.; Shervani, Z.; Dutta, A.K.; Izawa, H.; Morimoto, M.; Saimoto, H. Preparation of chitin nanofiber-reinforced double-decker-shaped polysilsesquioxane film. J. Chitin Chitosan Sci. 2013, 1, 1–5. [Google Scholar] [CrossRef]
- Ifuku, S.; Ikuta, A.; Egusa, M.; Kaminaka, H.; Izawa, H.; Morimoto, M.; Saimoto, H. Preparation of high-strength transparent chitosan film reinforced with surface-deacetylated chitin nanofibers. Carbohydr. Polym. 2013, 98, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Ikuta, A.; Izawa, H.; Morimoto, M.; Saimoto, H. Control of mechanical properties of chitin nanofiber film using glycerol without losing its characteristics. Carbohydr. Polym. 2014, 101, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Nakagaito, A.N.; Yamada, K.; Ifuku, S.; Morimoto, M.; Saimoto, H. Fabrication of chitin nanofiber-reinforced polylactic acid nanocomposites by an environmentally friendly process. J. Biobased Mater. Bioenergy 2013, 7, 152–156. [Google Scholar] [CrossRef]
- Ding, F.; Deng, H.; Du, Y.; Shi, X.; Wang, Q. Emerging chitin and chitosan nanofibrous materials for biomedical applications. Nanoscale 2014, 6, 9477–9493. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, N.; Hu, X.; Yang, J.; Du, Y. Chitosan/starch fibers and their properties for drug controlled release. Eur. J. Pharm. Biopharm. 2007, 66, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, N.; Hu, X.; Yang, J.; Du, Y. Chitosan/polyethylene glycol blend fibers and their properties for drug controlled release. J. Biomed. Mater. Res. A 2008, 85, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.I.; Mok, J.Y.; Jeon, I.H.; Park, K.-H.; Nguyen, T.T.T.; Park, J.S.; Hwang, H.M.; Song, M.S.; Lee, D.; Chai, K.Y. Effect of electrospun non-woven mats of dibutyryl chitin/poly(lactic acid) blends on wound healing in hairless mice. Molecules 2012, 17, 2992–3007. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Osaki, T.; Ifuku, S.; Saimoto, H.; Morimoto, M.; Takashima, O.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Minami, S. Anti-inflammatory effects of cellulose nanofiber made from pear in inflammatory bowel disease model. Bioact. Carbohydr. Diet. Fibre 2014, 3, 1–10. [Google Scholar] [CrossRef]
- Azuma, K.; Osaki, T.; Wakuda, T.; Ifuku, S.; Saimoto, H.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Minami, S. Beneficial and preventive effect of chitin nanofibrils in a dextran sulfate sodium-induced acute ulcerative colitis model. Carbohydr. Polym. 2012, 87, 1399–1403. [Google Scholar] [CrossRef]
- Azuma, K.; Osaki, T.; Ifuku, S.; Saimoto, H.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Minami, S. α-Chitin nanofibrils improve inflammatory and fibrosis responses in inflammatory bowel disease mice model. Carbohydr. Polym. 2012, 90, 197–200. [Google Scholar] [CrossRef]
- Ito, I.; Osaki, T.; Ifuku, S.; Saimoto, H.; Takamori, Y.; Kurozumi, S.; Imagawa, T.; Azuma, K.; Tsuka, T.; Okamoto, Y.; et al. Evaluation of the effects of chitin nanofibrils on skin function using skin models. Carbohydr. Polym. 2014, 101, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Ito, I.; Osaki, T.; Tokuda, K.; Asami, T.; Takamori, Y.; Kurozum, S.; Ifuku, S.; Saimoto, H.; Imagawa, T.; Azuma, K.; et al. Effect of chitin nanofibril combined in rayon animal bedding on hairless mouse skin and on a three dimensional culture human skin model. J. Chitin Chitosan Sci. 2014, 2, 82–88. [Google Scholar] [CrossRef]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ifuku, S. Chitin and Chitosan Nanofibers: Preparation and Chemical Modifications. Molecules 2014, 19, 18367-18380. https://doi.org/10.3390/molecules191118367
Ifuku S. Chitin and Chitosan Nanofibers: Preparation and Chemical Modifications. Molecules. 2014; 19(11):18367-18380. https://doi.org/10.3390/molecules191118367
Chicago/Turabian StyleIfuku, Shinsuke. 2014. "Chitin and Chitosan Nanofibers: Preparation and Chemical Modifications" Molecules 19, no. 11: 18367-18380. https://doi.org/10.3390/molecules191118367