Pterocarpan-Enriched Soy Leaf Extract Ameliorates Insulin Sensitivity and Pancreatic ?-Cell Proliferation in Type 2 Diabetic Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. EASL Lowered Body Weight Gain and Abdominal White Adipose Tissue (WAT) Weight
| Parameter | ND | HFD | EASL | Pinitol |
|---|---|---|---|---|
| Body weight (g) | ||||
| Initial | 20.3 ± 0.6 a | 19.5 ± 0.2 a | 20.1 ± 0.3 a | 19.6 ± 0.3 a |
| Final | 26.8 ± 0.6 c | 40.2 ± 0.8 a | 36.3 ± 0.7 b | 36.7 ± 0.4 b |
| Weight gain (g/12 week) | 6.5 ± 0.2 c | 20.7 ± 0.8 a | 16.3 ± 0.6 b | 17.0 ± 0.2 b |
| Food intake (g/day) | 2.5 ± 0.1 a | 2.1 ± 0.1 b | 2.1 ± 0.1 ab | 2.2 ± 0.1 ab |
| Organ weight (g) | ||||
| Abdominal adipose tissue | 0.26 ± 0.02 c | 2.01 ± 0.07 a | 1.50 ± 0.13 b | 1.80 ± 0.10 ab |
| Pancreas | 0.14 ± 0.02 a | 0.15 ± 0.03 a | 0.19 ± 0.01 a | 0.17 ± 0.01 a |
| Liver | 0.81 ± 0.01 b | 1.03 ± 0.04 a | 0.95 ± 0.02 a | 0.97 ± 0.01 a |
| Muscle | 0.31 ± 0.01 a | 0.31 ± 0.01 a | 0.31 ± 0.04 a | 0.32 ± 0.01 a |

2.2. Effect of EASL on Plasma Biochemical Parameters
| Parameter | ND | HFD | EASL | Pinitol |
|---|---|---|---|---|
| Glucose (mg/dL) | 93.9 ± 3.1 c | 203.7 ± 3.9 a | 193.0 ± 6.0 ab | 171.2 ± 9.1 b |
| HbAlc (%) | 4.6 ± 0.1 b | 5.4 ± 0.2 a | 4.5 ± 0.1 b | 4.5 ± 0.1 b |
| Insulin (ng/mL) | 0.5 ± 0.0 b | 1.5 ± 0.2 a | 0.9 ± 0.1 b | 0.9 ± 0.1 b |
| HOMA-IR index | 3.0 ± 0.1 b | 13.8 ± 2.5 a | 11.3 ± 1.1 a | 9.1 ± 1.9 ab |
| TC (mg/dL) | 149.7 ± 5.2 ab | 168.4 ± 5.6 a | 136.3 ± 11.0 b | 98.2 ± 9.6 c |
| HDL-C/TC (%) | 0.44 ± 0.05 a | 0.36 ± 0.08 a | 0.56 ± 0.10 a | 0.58 ± 0.04 a |
| TG (mg/dL) | 20.8 ± 3.9 b | 67.6 ± 11.4 a | 54.3 ± 9.1 a | 50.0 ± 5.4 a |
| GOT (IU/L) | 113.8 ± 10.0 a | 120.0 ± 8.1 a | 72.1 ± 1.2 b | 67.0 ± 1.9 b |
| GPT (IU/L) | 68.9 ± 4.7 ab | 71.4 ± 6.3 a | 54.8 ± 1.9 bc | 50.5 ± 2.3 c |
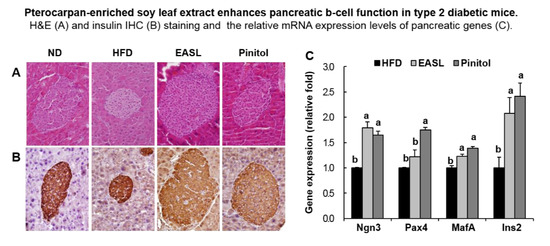
2.3. Effect of EASL on Pancreatic Function


2.4. Effect of EASL on Expression of Gluconeogenesis and Insulin Sensitivity-Related Genes in Liver Tissue and WAT

2.5. Discussion
3. Experimental Section
3.1. Preparation of Soy Leaf Extracts
3.2. HPLC Analysis of EASL
3.3. Animals and Diets

3.4. Measurement of Metabolic Parameters
3.5. Histological Analysis of WAT, Liver, and Pancreas
3.6. IHC Analysis of the Pancreas
3.7. Pancreatic Insulin Content
3.8. Real-Time Quantitative RT-PCR (qRT-PCR)
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| C/EBPα | CAAGAAGTCGGTGGACAAGA | TCAACTCCAGCACCTTCTGT |
| FoxO1 | TGGGCCCTAATTCGGTCAT | TTGGGTCAGGCGGTTCATAC |
| GLUT4 | GCCCCACAGAAGGTGATTGA | AGCGTAGTGAGGGTGCCTTGT |
| G6Pase | GCTGGAGTCTTGTCAGGCATT | AAACAAGAAGATGGTGATGAGACAAT |
| GAPDH | ACATCATCCCTGCATCCACT | AGATCCACGACGGACACATT |
| Ins2 | CCCCACCCAGGCTTTTGT | GCGGGACATGGGTGTGTAG |
| Insr | CTGAACAAAGATGACAACGAGGAA | CTTACAGATGGTTGGGCAAACTT |
| IRS1 | GAGAAGAGACTGGCTCGGAAGA | GCCTATTCTGCCCAACTCAACT |
| IRS2 | GGCCCGAACCTCAATAACAA | CCGCGCAACACGAAAAAG |
| IL-1β | ATGAGGACATGAGCACCTTC | CATTGAGGTGGAGAGCTTTC |
| IL-6 | GCTACCTGGAGTACATGAAG | CTGTGACTCCAGCTTATCTG |
| MafA | GAACCGGAGGAGAATAAGAGG | AGTCAAGTTGAGCATCACTGC |
| Ngn3 | CACTCAGCAAACAGCGAAGAAG | GTCAGTGCCCAGATGTAGTTGTG |
| p53 | ACATCATCCCTGCATCCACT | AGATCCACGACGGACACATT |
| Pax4 | CCCAGTGTGTCCTCTATCAATCG | GGGAAGAACTGGAGCCAACA |
| PEPCK | AGACCCTGCGAGTGCTTAGTG | AGGGTCAGTGTGGCAGTATTCTC |
| PKA | GGTTTTGCCAAGCGTGTGA | CAGCCTTGTTGTAGCCTTTGC |
| TNFα | CTCAGATCATCTTCTCAAAATTCGAGTGACA | CTTCACAGAGCAATGACTCCAAAGT |
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Ehses, J.A.; Maedler, K.; Schumann, D.M.; Ellingsgaard, H.; Eppler, E.; Reinecke, M. Mechanisms of β-Cell Death in Type 2 Diabetes. Diabetes 2005, 54, S108–S113. [Google Scholar] [CrossRef] [PubMed]
- Leto, D.; Saltiel, A.R. Regulation of glucose transport by insulin: Traffic control of GLUT4. Nat. Rev. Mol. Cell Biol. 2012, 13, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Thong, F.S.; Dugani, C.B.; Klip, A. Turning signals on and off: GLUT4 traffic in the insulin-signaling highway. Physiology 2005, 20, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Copps, K.D.; White, M.F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012, 55, 2565–2582. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. Mechanisms for insulin resistance: Common threads and missing links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; Barnes, S. The role of soy products in reducing risk of cancer. J. Natl. Cancer Inst. 1991, 83, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.; Yu, M.C.; Tseng, C.C.; Pike, M.C. Epidemiology of soy exposures and breast cancer risk. Br. J. Cancer 2008, 98, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Takatsuka, N.; Kurisu, Y.; Shimizu, H. Decreased serum total cholesterol concentration is associated with high intake of soy products in Japanese men and women. J. Nutr. 1998, 128, 209–213. [Google Scholar] [PubMed]
- Jenkins, D.J.; Mirrahimi, A.; Srichaikul, K.; Berryman, C.E.; Wang, L.; Carleton, A.; Abdulnour, S.; Sievenpiper, J.L.; Kendall, C.W.; Kris-Etherton, P.M. Soy protein reduces serum cholesterol by both intrinsic and food displacement mechanisms. J. Nutr. 2010, 140, 2302S–2311S. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health & Human Services, Food and Drug Administration. Food Labeling: Health Claims; Soy Protein and Coronary Heart Disease; Federal Register: Washington, DC, USA, 1999; Volume 64, pp. 57700–57733.
- Sacks, F.M.; Lichtenstein, A.; van Horn, L.; Harris, W.; Kris-Etherton, P.; Winston, M. Soy protein, isoflavones, and cardiovascular health: An American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation 2006, 113, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Guo, M.; Hua, Y.; Cao, D.; Zhang, C. Enzymatic preparation of immunomodulating hydrolysates from soy proteins. Bioresour. Technol. 2008, 99, 8873–8879. [Google Scholar] [CrossRef] [PubMed]
- Masotti, A.I.; Buckley, N.; Champagne, C.P.; Green-Johnson, J. Immunomodulatory bioactivity of soy and milk ferments on monocyte and macrophage models. Food Res. Int. 2011, 44, 2475–2481. [Google Scholar] [CrossRef]
- Setchell, K.D. Phytoestrogens: The biochemistry, physiology, and implications for human health of soy isoflavones. Am. J. Clin. Nutr. 1998, 68, 1333S–1346S. [Google Scholar] [PubMed]
- Ho, H.M.; Chen, R.Y.; Leung, L.K.; Chan, F.L.; Huang, Y.; Chen, Z.Y. Difference in flavonoid and isoflavone profile between soybean and soy leaf. Biomed. Pharmacother. 2002, 56, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.J.; Lee, J.H.; Curtis-Long, M.J.; Lee, J.W.; Kim, Y.S.; Ryu, H.W.; Park, C.G.; Jeong, T.S.; Park, K.H. The most abundant polyphenol of soy leaves, coumestrol, displays potent α-glucosidase inhibitory activity. Food Chem. 2011, 126, 1057–1063. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, B.W.; Kim, J.H.; Jeong, T.S.; Kim, M.J.; Lee, W.S.; Park, K.H. LDL-antioxidant pterocarpans from roots of Glycine max (L.) Merr. J. Agric. Food Chem. 2006, 54, 2057–2063. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.J.; Curtis-Long, M.J.; Ryu, H.W.; Jang, K.C.; Seo, W.D.; Kim, J.Y.; Kang, K.Y.; Park, K.H. Pterocarpan profiles for soybean leaves at different growth stages and investigation of their glycosidase inhibitions. J. Agric. Food Chem. 2011, 59, 12683–12690. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Sandjo, L.P.; Kwamou, G.M.; Wiench, B.; Nkengfack, A.E.; Efferth, T. Activity of three cytotoxic isoflavonoids from Erythrina excelsa and Erythrina senegalensis (neobavaisoflavone, sigmoidin H and isoneorautenol) toward multi-factorial drug resistant cancer cells. Phytomedicine 2014, 21, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hsieh, D.; Yang, Y.L.; Xu, Z.; Peto, C.; Jablons, D.M.; You, L. Coumestrol from the national cancer Institute’s natural product library is a novel inhibitor of protein kinase CK2. BMC Pharmacol. Toxicol. 2013, 11, 14–36. [Google Scholar]
- Jantaratnotai, N.; Utaisincharoen, P.; Sanvarinda, P.; Thampithak, A.; Sanvarinda, Y. Phytoestrogens mediated anti-inflammatory effect through suppression of IRF-1 and pSTAT1 expressions in lipopolysaccharide-activated microglia. Int. Immunopharmacol. 2013, 17, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Larner, J.; Brautigan, D.L.; Thorner, M.O. D-chiro-inositol glycans in insulin signaling and insulin resistance. Mol. Med. 2010, 16, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Kou, K.; Saisho, Y.; Sato, S.; Yamada, T.; Itoh, H. Islet number rather than silet is a major determinant of β-cell and α-cell mass in humans. J. Clin. Endocrinol. Metab. 2014, 99, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Peyot, M.L.; Pepin, E.; Lamontagne, J.; Latour, M.G.; Zarrouki, B.; Lussier, R.; Pineda, M.; Jetton, T.L.; Madiraju, S.R.; Joly, E.; et al. Beta-cell failure in diet-induced obese mice stratified according to body weight gain: secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta-cell mass. Diabetes 2010, 59, 2178–2187. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Ryu, R.; Seo, Y.R.; Jeong, T.S.; Shin, D.H.; Park, Y.B.; Kim, S.R.; Jung, U.J. The beneficial effect of soybean (Glycine max (L.) Merr.) leaf extracts in adults with prediabetes: A randomized placebo controlled trial. Food Funct. 2014, 25, 1621–1630. [Google Scholar] [CrossRef]
- Kim, J.E.; Jeon, S.M.; Park, K.H.; Lee, W.S.; Jeong, T.S.; McGregor, R.A.; Choi, M.S. Does Glycine max leaves or Garcinia Cambogia promote weight-loss or lower plasma cholesterol in overweight individuals: a randomized control trial. Nutr. J. 2011, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, S.K.; Rahamathulla, S.; Bardia, A.; Tiwari, S.K.; Srinivas, G.; Raj, A.; Tripura, C.; Sandhya, A.; Habeeb, M.A.; Khan, A.A.; et al. In vitro quantitative and relative gene expression analysis of pancreatic transcription factors Pdx-1, Ngn-3, Isl-1, Pax-4, Pax-6 and Nkx-6.1 in trans-differentiated human hepatic progenitors. J. Diabetes Investig. 2014, 5, 492–500. [Google Scholar] [CrossRef]
- Kaneto, H.; Miyatsuka, T.; Fujitani, Y.; Noguchi, H.; Song, K.H.; Yoon, K.H.; Matsuoka, T.A. Role of PDX-1 and MafA as a potential therapeutic target for diabetes. Diabetes Res. Clin. Pract. 2007, 77, S127–S137. [Google Scholar] [CrossRef] [PubMed]
- Vetere, A.; Choudhary, A.; Burns, S.M.; Wagner, B.K. Targeting the pancreatic β-cell to treat diabetes. Nat. Rev. Drug Discov. 2014, 13, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Al-Hasani, K.; Pfeifer, A.; Courtney, M.; Ben-Othman, N.; Gjernes, E.; Vieira, A.; Druelle, N.; Avolio, F.; Ravassard, P.; Leuckx, G.; et al. Adult duct-lining cells can reprogram into β-like cells able to counter repeated cycles of toxin-induced diabetes. Dev. Cell 2013, 26, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Leibiger, I.B.; Leibiger, B.; Berggren, P.O. Insulin signaling in the pancreatic beta-cell. Annu. Rev. Nutr. 2008, 28, 233–251. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.H.; Jones, R.B.; Bailey, C.J. Insulin-like effect of pinitol. Br. J. Pharmacol. 2000, 130, 1944–1948. [Google Scholar] [PubMed]
- Davis, A.; Christiansen, M.; Horowitz, J.F.; Klein, S.; Hellerstein, M.K.; Ostlund, R.E., Jr. Effect of pinitol treatment on insulin action in subjects with insulin resistance. Diabetes Care 2000, 23, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Geethan, P.K.; Prince, P.S. Antihyperlipidemic effect of D-pinitol on streptozotocin-induced diabetic Wistar rats. J. Biochem. Mol. Toxicol. 2008, 22, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Kim, J.C.; Kang, M.J.; Lee, M.S.; Kim, J.J.; Cha, I.J. Effects of pinitol isolated from soybeans on glycaemic control and cardiovascular risk factors in Korean patients with type II diabetes mellitus: a randomized controlled study. Eur. J. Clin. Nutr. 2005, 59, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, S.; Palsamy, P.; Subramanian, S.P. Impact of D-pinitol on the attenuation of proinflammatory cytokines, hyperglycemia-mediated oxidative stress and protection of kidney tissue ultrastructure in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2010, 188, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Kahn, C.R. Insulin signaling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Altarejos, J.Y.; Montminy, M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. 2011, 12, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Accili, D. The tangled path to glucose production. Nat. Med. 2006, 12, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Jitrapakdee, S. Transcription factors and coactivators controlling nutrient and hormonal regulation of hepatic gluconeogenesis. Int. J. Biochem. Cell Biol. 2012, 44, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, O.P.; Mandrup-Poulsen, T. Interleukin-6 and diabetes: The good, the bad, or the indifferent? Diabetes 2005, 54, S114–S124. [Google Scholar] [CrossRef]
- Permana, P.A.; Menge, C.; Reaven, P.D. Macrophage-secreted factors induce adipocyte inflammation and insulin resistance. Biochem. Biophys. Res. Commun. 2006, 341, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Ethyl acetate extracts of soy leaves (EASL) are available from the corresponding author Tae-Sook Jeong.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, U.-H.; Yoon, J.-H.; Li, H.; Kang, J.-H.; Ji, H.-S.; Park, K.H.; Shin, D.-H.; Park, H.-Y.; Jeong, T.-S. Pterocarpan-Enriched Soy Leaf Extract Ameliorates Insulin Sensitivity and Pancreatic ?-Cell Proliferation in Type 2 Diabetic Mice. Molecules 2014, 19, 18493-18510. https://doi.org/10.3390/molecules191118493
Kim U-H, Yoon J-H, Li H, Kang J-H, Ji H-S, Park KH, Shin D-H, Park H-Y, Jeong T-S. Pterocarpan-Enriched Soy Leaf Extract Ameliorates Insulin Sensitivity and Pancreatic ?-Cell Proliferation in Type 2 Diabetic Mice. Molecules. 2014; 19(11):18493-18510. https://doi.org/10.3390/molecules191118493
Chicago/Turabian StyleKim, Un-Hee, Jeong-Hyun Yoon, Hua Li, Ji-Hyun Kang, Hyeon-Seon Ji, Ki Hun Park, Dong-Ha Shin, Ho-Yong Park, and Tae-Sook Jeong. 2014. "Pterocarpan-Enriched Soy Leaf Extract Ameliorates Insulin Sensitivity and Pancreatic ?-Cell Proliferation in Type 2 Diabetic Mice" Molecules 19, no. 11: 18493-18510. https://doi.org/10.3390/molecules191118493
APA StyleKim, U.-H., Yoon, J.-H., Li, H., Kang, J.-H., Ji, H.-S., Park, K. H., Shin, D.-H., Park, H.-Y., & Jeong, T.-S. (2014). Pterocarpan-Enriched Soy Leaf Extract Ameliorates Insulin Sensitivity and Pancreatic ?-Cell Proliferation in Type 2 Diabetic Mice. Molecules, 19(11), 18493-18510. https://doi.org/10.3390/molecules191118493