Anti-Proliferative Effects of Siegesbeckia orientalis Ethanol Extract on Human Endometrial RL-95 Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Inhibitory Effect of SOE on RL95-2 Cell Proliferation

2.2. Apoptotic Effects of SOE on RL95-2 Cells
2.2.1. Cell Morphology
2.2.2. Cell Cycle Regulation
2.2.3. Pro-Apoptotic and Anti-Apoptotic Proteins Expression



2.3. Cytotoxicity of SOE on Various Cancer Cell Lines
| Cell Type | Cell Line | IC50 (μg/mL) b |
|---|---|---|
| Human endometrial carcinoma | RL95-2 | 163.5 ± 3.3 |
| Human lung carcinoma | A549 | 179.1 ± 1.8 |
| Human hepatoblastoma | Hep G2 | 135.4 ± 2.4 |
| Human pharynx squamous cell carcinoma | FaDu | 105.0 ± 1.8 |
| Human breast adenocarcinoma | MDA-MB-231 | 124.3 ± 4.0 |
| Human prostate carcinoma | LNCaP | 87.2 ± 1.3 |
2.4. Cytotoxicity of Main Ingredients of SOE on RL95-2 Cells
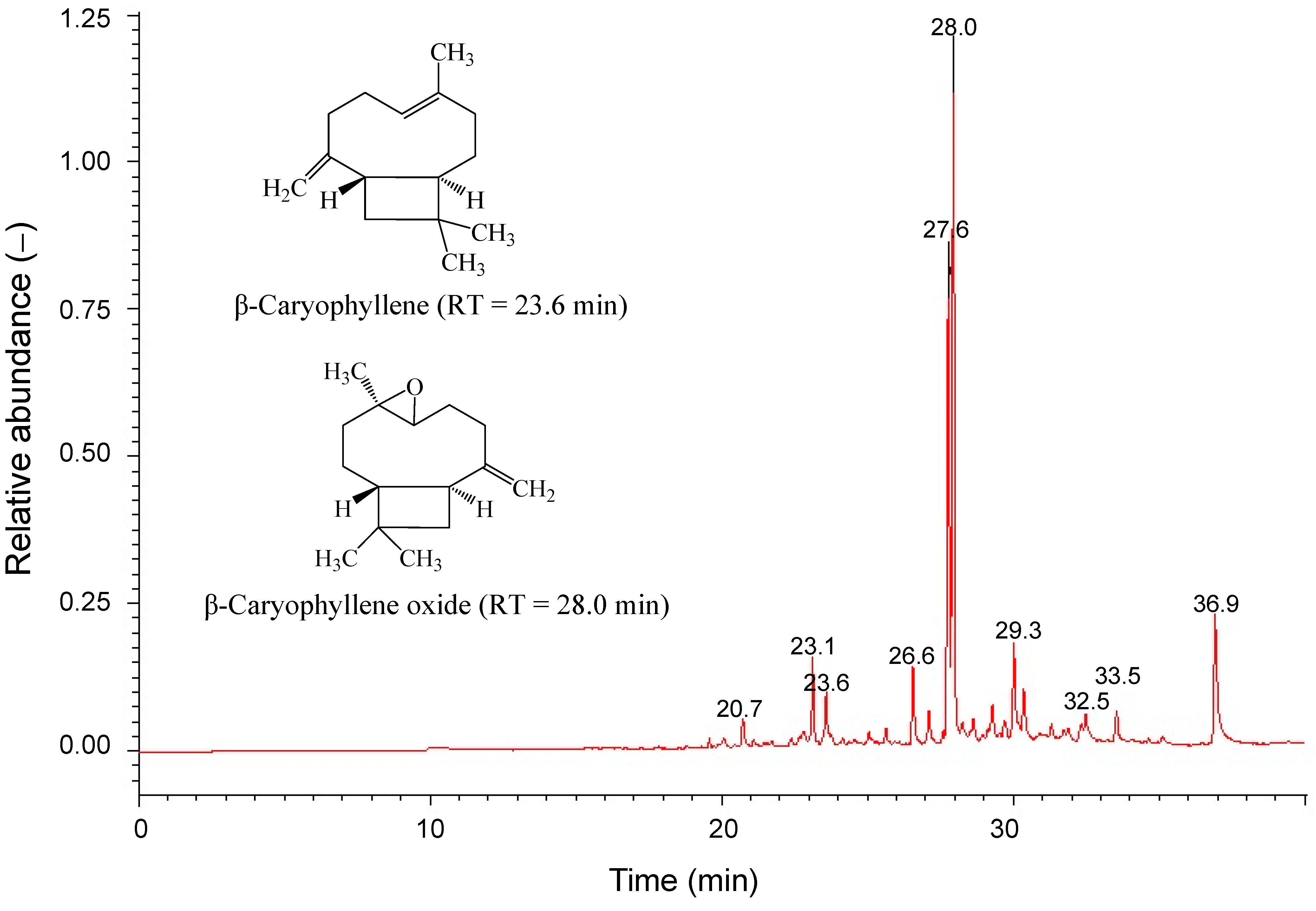
| No. | Component | Rt (min) a | Percentage (%) b | R. Match |
|---|---|---|---|---|
| 1 | 2-Oxabicyclo[2,2,2]octane-6-ol | 20.72 | 1.8 | 763 |
| 2 | 2-tert-Butyl-1,4-dimethoxy-benzene | 23.13 | 3.8 | 805 |
| 3 | Caryophyllene | 23.59 | 3.1 | 866 |
| 4 | cis-α-Bisabolene | 26.57 | 4.1 | 860 |
| 5 | [−]-Spathulenol | 27.62 | 25.7 | 853 |
| 6 | Caryophyllene oxide | 27.95 | 46.9 | 858 |
| 7 | cis-Lanceol | 29.28 | 1.7 | 719 |
| 8 | [Z,Z,Z]-9,12,15-Octadecatrienoic acid ethyl ester | 32.49 | 1.2 | 736 |
| 9 | 6,10,14-Trimethyl-2-pentadecanone | 33.53 | 2.1 | 790 |
| 10 | Hexadecanoic acid ethyl ester | 36.93 | 9.6 | 794 |
3. Experimental Section
3.1. Reagents and Materials
3.2. Preparation of S. orientalis Ethanol Extract
3.3. Cancer Cell Lines and Culture
3.4. Determination of Cytotoxicity for Cancer Cells
3.5. Flow Cytometry Analysis of Cell Cycle
3.6. Apoptotic Ratio Analysis
3.7. Preparation of Whole-Cell Lysates
3.8. Western Blot Analysis
3.9. Gas Chromatography-Mass Spectrometry
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mueck, A.O.; Seeger, H. The World Health Organization defines hormone replacement therapy as carcinogenic: Is this plausible? Gynecol. Endocrinol. 2008, 24, 129–132. [Google Scholar]
- Lheureux, S.; Joly, F. Endometrial cancer: Place for adjuvant chemotherapy. Bull. Cancer 2012, 99, 85–91. [Google Scholar]
- Lau, H.Y.; Chen, Y.J.; Yen, M.S.; Chao, K.C.; Chen, R.F.; Yeh, S.O.; Twu, N.F. Clinicopathological features and survival in young taiwanese women with endometrial carcinoma. Int. J. Gynecol. Cancer 2014, 24, 1015–1020. [Google Scholar]
- Kaaks, R.; Lukanova, A.; Kurzer, M.S. Obesity, endogenous hormones, and endometrial cancer risk: A synthetic review. Cancer Epidemiol. Biomarker. Prev. 2002, 11, 1531–1543. [Google Scholar]
- Plataniotis, G.; Castiglione, M. Endometrial cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. nn. Oncol. 2010, 21 (Suppl. 5), v41–v45. [Google Scholar]
- Mu, N.; Zhu, Y.; Wang, Y.; Zhang, H.; Xue, F. Insulin resistance: A significant risk factor of endometrial cancer. Gynecol. Oncol. 2012, 125, 751–757. [Google Scholar]
- Schmandt, R.E.; Iglesias, D.A.; Co, N.N.; Lu, K.H. Understanding obesity and endometrial cancer risk: Opportunities for prevention. Am. J. Obstet. Gynecol. 2011, 205, 518–525. [Google Scholar]
- Lee, N.K. Adjuvant treatment of advanced-stage endometrial cancer. Clin. Obstet. Gynecol. 2011, 54, 256–265. [Google Scholar]
- Bouchard, P. Current and future medical treatments for menometrorrhagia during the premenopause. Gynecol. Endocrinol. 2011, 27, 1120–1125. [Google Scholar]
- Liu, J.; Li, X.; Ma, L.; Fonnebo, V. Traditional Chinese medicine in cancer care: A review of case reports published in Chinese literature. Forsch. Komplement. Med. 2011, 18, 257–263. [Google Scholar]
- Efferth, T. Personalized cancer medicine: From molecular diagnostics to targeted therapy with natural products. Planta Med. 2010, 76, 1143–1154. [Google Scholar]
- Mehta, R.G.; Murillo, G.; Naithani, R.; Peng, X. Cancer chemoprevention by natural products: How far have we come? Pharm. Res. 2010, 27, 950–961. [Google Scholar]
- Wang, F.; Cheng, X.L.; Li, Y.J.; Shi, S.; Liu, J.K. ent-Pimarane diterpenoids from Siegesbeckia orientalis and structure revision of a related compound. J. Nat. Prod. 2009, 72, 2005–2008. [Google Scholar]
- Qian, R.Q.; Zhang, C.Y.; Fu, H.Z. Study on therapeutic mechanism of anti-rheumatism action of Herba siegesbeckiae. Chin. J. Integr. Tradit. West. Med. 2000, 20, 192–195. [Google Scholar]
- Wang, J.P.; Zhou, Y.M.; Ye, Y.J.; Shang, X.M.; Cai, Y.L.; Xiong, C.M.; Wu, Y.X.; Xu, H.X. Topical anti-inflammatory and analgesic activity of kirenol isolated from Siegesbeckia orientalis. J. Ethnopharmacol. 2011, 137, 1089–1094. [Google Scholar]
- Hwang, W.J.; Park, E.J.; Jang, C.H.; Han, S.W.; Oh, G.J.; Kim, N.S.; Kim, H.M. Inhibitory effect of immunoglobulin E production by jin-deuk-chal (Siegesbeckia orientalis). Immunopharmacol. Immunotoxicol. 2001, 23, 555–563. [Google Scholar]
- Sun, H.X.; Wang, H. Immunosuppressive activity of the ethanol extract of Siegesbeckia orientalis on the immune responses to ovalbumin in mice. Chem. Biodivers. 2006, 3, 754–761. [Google Scholar]
- Wang, J.P.; Luo, Q.; Ruan, J.L.; Chen, Y.A.; Chen, M.X. Effect of Siegesbeckia orientalis L. on cervical cancer HeLa cell in vitro. Herald Med. 2009, 28, 45–46. (In Chinese) [Google Scholar]
- Cotter, T.G. Apoptosis and cancer: The genesis of a research field. Nat. Rev. Cancer 2009, 9, 501–507. [Google Scholar]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. Biomed. Res. Int. 2014, 1–23. [Google Scholar]
- Wyllie, A.H.; Kerr, J.F.; Currie, A.R. Cell death: The significance of apoptosis. Int. Rev. Cytol. 1980, 68, 251–306. [Google Scholar]
- Mohan, S.; Abdul, A.B.; Abdelwahab, S.I.; Al-Zubairi, A.S.; Sukari, M.A.; Abdullah, R.; Taha, M.M.; Beng, N.K.; Isa, N.M. Typhonium flagelliforme inhibits the proliferation of murine leukemia WEHI-3 cells in vitro and induces apoptosis in vivo. Leuk. Res. 2010, 34, 1483–1492. [Google Scholar]
- Hsu, H.F.; Huang, K.H.; Lu, K.J.; Chiou, S.J.; Yen, J.H.; Chang, C.C.; Houng, J.Y. Typhonium blumei extract inhibits proliferation of human lung adenocarcinoma A549 cells via induction of cell cycle arrest and apoptosis. J. Ethnopharmacol. 2011, 135, 492–500. [Google Scholar]
- Yoon, J.H.; Gores, G.J. Death receptor-mediated apoptosis and the liver. J. Hepatol. 2002, 37, 400–410. [Google Scholar]
- Catz, S.D.; Johnson, J.L. BCL-2 in prostate cancer: A mini review. Apoptosis 2003, 8, 29–37. [Google Scholar]
- Shirazi, M.T.; Gholami, H.; Kavoosi, G.; Rowshan, V.; Tafsiry, A. Chemical composition, antioxidant, antimicrobial and cytotoxic activities of Tagetes minuta and Ocimum basilicum essential oils. Food Sci. Nutr. 2014, 2, 146–155. [Google Scholar]
- Wu, J.G.; Peng, W.; Yi, J.; Wu, Y.B.; Chen, T.Q.; Wong, K.H.; Wu, J.Z. Chemical composition, antimicrobial activity against Staphylococcus aureus and a pro-apoptotic effect in SGC-7901 of the essential oil from Toona sinensis (A. Juss.) Roem. leaves. J. Ethnopharmacol. 2014, 154, 198–205. [Google Scholar]
- Bayala, B.; Bassole, I.H.; Gnoula, C.; Nebie, R.; Yonli, A.; Morel, L.; Figueredo, G.; Nikiema, J.B.; Lobaccaro, J.M.; Simpore, J. Chemical composition, antioxidant, anti-inflammatory and anti-proliferative activities of essential oils of plants from Burkina faso. PLoS One 2014, 9, e92122. [Google Scholar]
- Lampronti, I.; Saab, A.M.; Gambari, R. Antiproliferative activity of essential oils derived from plants belonging to the Magnoliophyta division. Int. J. Oncol. 2006, 29, 989–995. [Google Scholar]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F.; Dodaro, D.; Passalacqua, N.G.; Statti, G.; Menichini, F. In vitro cytotoxic effects of Senecio stabianus Lacaita (Asteraceae) on human cancer cell lines. Nat. Prod. Res. 2009, 23, 1707–1718. [Google Scholar]
- Su, Y.C.; Hsu, K.P.; Wang, E.I.; Ho, C.L. Composition and in vitro anticancer activities of the leaf essential oil of Neolitsea variabillima from Taiwan. Nat. Prod. Commun. 2013, 8, 531–532. [Google Scholar]
- Park, K.R.; Nam, D.; Yun, H.M.; Lee, S.G.; Jang, H.J.; Sethi, G.; Cho, S.K.; Ahn, K.S. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar]
- Ryu, N.H.; Park, K.R.; Kim, S.M.; Yun, H.M.; Nam, D.; Lee, S.G.; Jang, H.J.; Ahn, K.S.; Kim, S.H.; Shim, B.S.; et al. A hexane fraction of guava leaves (Psidium guajava L.) induces anticancer activity by suppressing AKT/mammalian target of rapamycin/ribosomal p70 S6 kinase in human prostate cancer cells. J. Med. Food 2012, 15, 231–241. [Google Scholar]
- Kim, C.; Cho, S.K.; Kapoor, S.; Kumar, A.; Vali, S.; Abbasi, T.; Kim, S.H.; Sethi, G.; Ahn, K.S. β-Caryophyllene oxide inhibits constitutive and inducible STAT3 signaling pathway through induction of the SHP-1 protein tyrosine phosphatase. Mol. Carcinog 2013. [Google Scholar] [CrossRef]
- Sain, S.; Naoghare, P.K.; Devi, S.S.; Daiwile, A.; Krishnamurthi, K.; Arrigo, P.; Chakrabarti, T. Beta caryophyllene and caryophyllene oxide, isolated from Aegle marmelos, as the potent anti-inflammatory agents against lymphoma and neuroblastoma cells. Antiinflamm. Antiallergy Agents Med. Chem. 2014, 13, 45–55. [Google Scholar]
- Oh, M.S.; Yang, J.Y.; Kim, M.G.; Lee, H.S. Acaricidal activities of β-caryophyllene oxide and structural analogues derived from Psidium cattleianum oil against house dust mites. Pest. Manag. Sci. 2014, 70, 757–762. [Google Scholar]
- Hsu, H.F.; Chang, S.F.; Chen, Z.H.; Yuan, S.S.F.; Tsai, Y.D.; Wang, C.P.; Wang, S.W.; Fang, L.W.; Houng, J.Y. Cytotoxic effect of Anisomeles indica extract on human pharynx squamous cancer cells. J. Med. Plants. Res. 2012, 6, 5002–5012. [Google Scholar]
- Sample Availability: Samples of the compounds β-caryophyllene and caryophyllene oxide are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-C.; Hsu, H.-F.; Huang, K.-H.; Wu, J.-M.; Kuo, S.-M.; Ling, X.-H.; Houng, J.-Y. Anti-Proliferative Effects of Siegesbeckia orientalis Ethanol Extract on Human Endometrial RL-95 Cancer Cells. Molecules 2014, 19, 19980-19994. https://doi.org/10.3390/molecules191219980
Chang C-C, Hsu H-F, Huang K-H, Wu J-M, Kuo S-M, Ling X-H, Houng J-Y. Anti-Proliferative Effects of Siegesbeckia orientalis Ethanol Extract on Human Endometrial RL-95 Cancer Cells. Molecules. 2014; 19(12):19980-19994. https://doi.org/10.3390/molecules191219980
Chicago/Turabian StyleChang, Chi-Chang, Hsia-Fen Hsu, Kuo-Hung Huang, Jing-Mei Wu, Shyh-Ming Kuo, Xue-Hua Ling, and Jer-Yiing Houng. 2014. "Anti-Proliferative Effects of Siegesbeckia orientalis Ethanol Extract on Human Endometrial RL-95 Cancer Cells" Molecules 19, no. 12: 19980-19994. https://doi.org/10.3390/molecules191219980





