Total Synthesis and Anti-Viral Activities of an Extract of Radix isatidis
Abstract
:1. Introduction
2. Results and Discussion
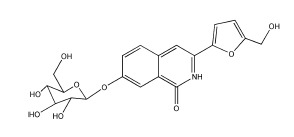
2.1. Chemistry
2.2. Biological Activity

| Compounds | IC50 (µg/mL) | TC50 (µg/mL) | TI * |
|---|---|---|---|
| 7 | >200 | - | - |
| 8 | 15.3 | 90.9 | 5.94 |
| 10 | 42.4 | 72.1 | 1.70 |
| 1 | 79.1 | 619.4 | 7.83 |
| Acyclovir | 1.4 | - | - |
3. Experimental Section
3.1. Chemistry
3.1.1. Materials and Solutions
3.1.2. Synthesis
3.2. Anti-Virus Biological Assay
3.2.1. Materials and Solutions
3.2.2. Cytotoxic Effects of Compounds on Vero Cells
3.2.3. Anti-HSV-1 Activity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, W.; Zhang, X.Y. Research progress of Chinese herbal medicine Radix isatidis (banlangen). Am. J. Chin. Med. 2013, 41, 743–764. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.F.; Lu, M.C.; Chang, H.C.; Wei, C.C.; Kao, C.H.; Chen, Z.H.; Huang, C.C.; Li, C. Effects of herbal medicinal medicinal formulas on suppressing viral replication and modulating immune responses. Am. J. Chin. Med. 2010, 38, 173–190. [Google Scholar] [CrossRef] [PubMed]
- He, L.W.; Dong, W.; Yang, J.Y.; Li, X.; Li, W. Chemical constituents in antiviral fraction of Isatidis Radix and their activities. Zhong Cao Yao 2013, 44, 2960–2964. [Google Scholar]
- He, L.W.; Li, X.; Chen, J.W. Research progress of Radix isatidis against virus. Inform. Tradit. Chin. Med. 2005, 22, 37–40. [Google Scholar]
- He, L.W.; Li, X.; Chen, J.W.; Sun, D.D.; Ju, W.Z.; Wang, K.C. Chemical constituents from water extract of Radix isatidis. Yao Xue Xue Bao 2006, 41, 1193–1196. [Google Scholar] [PubMed]
- Wang, T.L.; Xiang, H.; Xu, X.L.; Xiao, H.; You, Q.D. Synthesis and anti-cancer activities of novel 6-arylindenoisoquinolines. Chin. J. Med. Chem. 2012, 22, 268–274. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.-W.; Liu, H.-Q.; Chen, Y.-Q.; Yang, J.-Y.; Wang, T.-L.; Li, W. Total Synthesis and Anti-Viral Activities of an Extract of Radix isatidis. Molecules 2014, 19, 20906-20912. https://doi.org/10.3390/molecules191220906
He L-W, Liu H-Q, Chen Y-Q, Yang J-Y, Wang T-L, Li W. Total Synthesis and Anti-Viral Activities of an Extract of Radix isatidis. Molecules. 2014; 19(12):20906-20912. https://doi.org/10.3390/molecules191220906
Chicago/Turabian StyleHe, Li-Wei, Hua-Qing Liu, Yu-Qing Chen, Jing-Yan Yang, Tian-Lin Wang, and Wei Li. 2014. "Total Synthesis and Anti-Viral Activities of an Extract of Radix isatidis" Molecules 19, no. 12: 20906-20912. https://doi.org/10.3390/molecules191220906
APA StyleHe, L.-W., Liu, H.-Q., Chen, Y.-Q., Yang, J.-Y., Wang, T.-L., & Li, W. (2014). Total Synthesis and Anti-Viral Activities of an Extract of Radix isatidis. Molecules, 19(12), 20906-20912. https://doi.org/10.3390/molecules191220906