Toxicity of Evodiae fructus on Rat Liver Mitochondria: The Role of Oxidative Stress and Mitochondrial Permeability Transition
Abstract
:1. Introduction
2. Results
2.1. Quantification of Evodiamine, Rutaecarpine and Evodine by HPLC/UV
| Ingredient | Extract | |
|---|---|---|
| mg/g | % | |
| Evodiamine | 0.096 ± 0.011 | 0.009 ± 0.001 |
| Rutaecarpine | 0.044 ± 0.001 | 0.004 ± 0.001 |
| Evodine | 7.473 ± 0.241 | 0.747 ± 0.024 |

2.2. Effect of EF on Manganese Superoxide Dismutase (MnSOD), Glutathione (GSH), and Malondialdehyde (MDA)
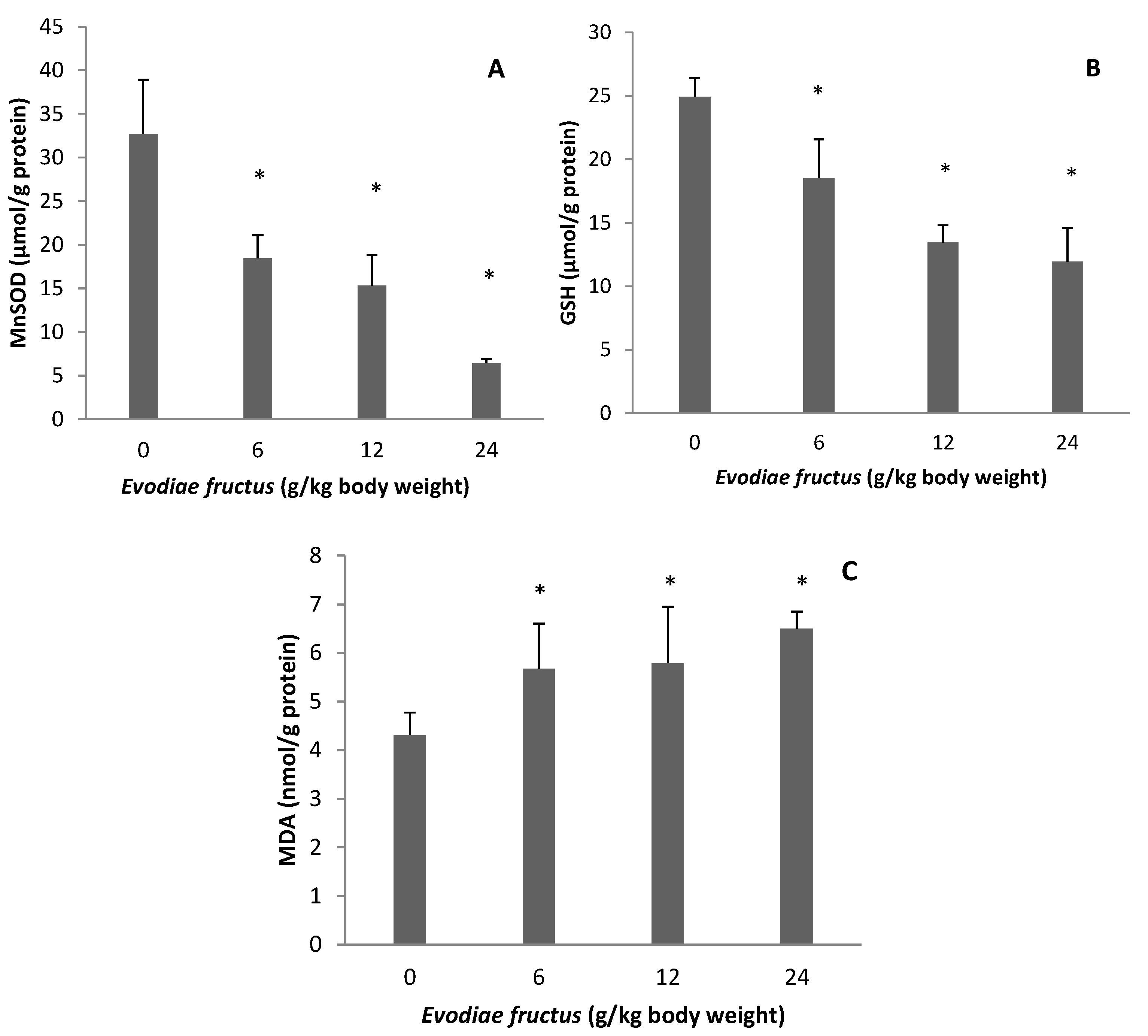
2.3. Effect on Mitochondrial Permeability Transition

2.4. Electron Microscopy Findings
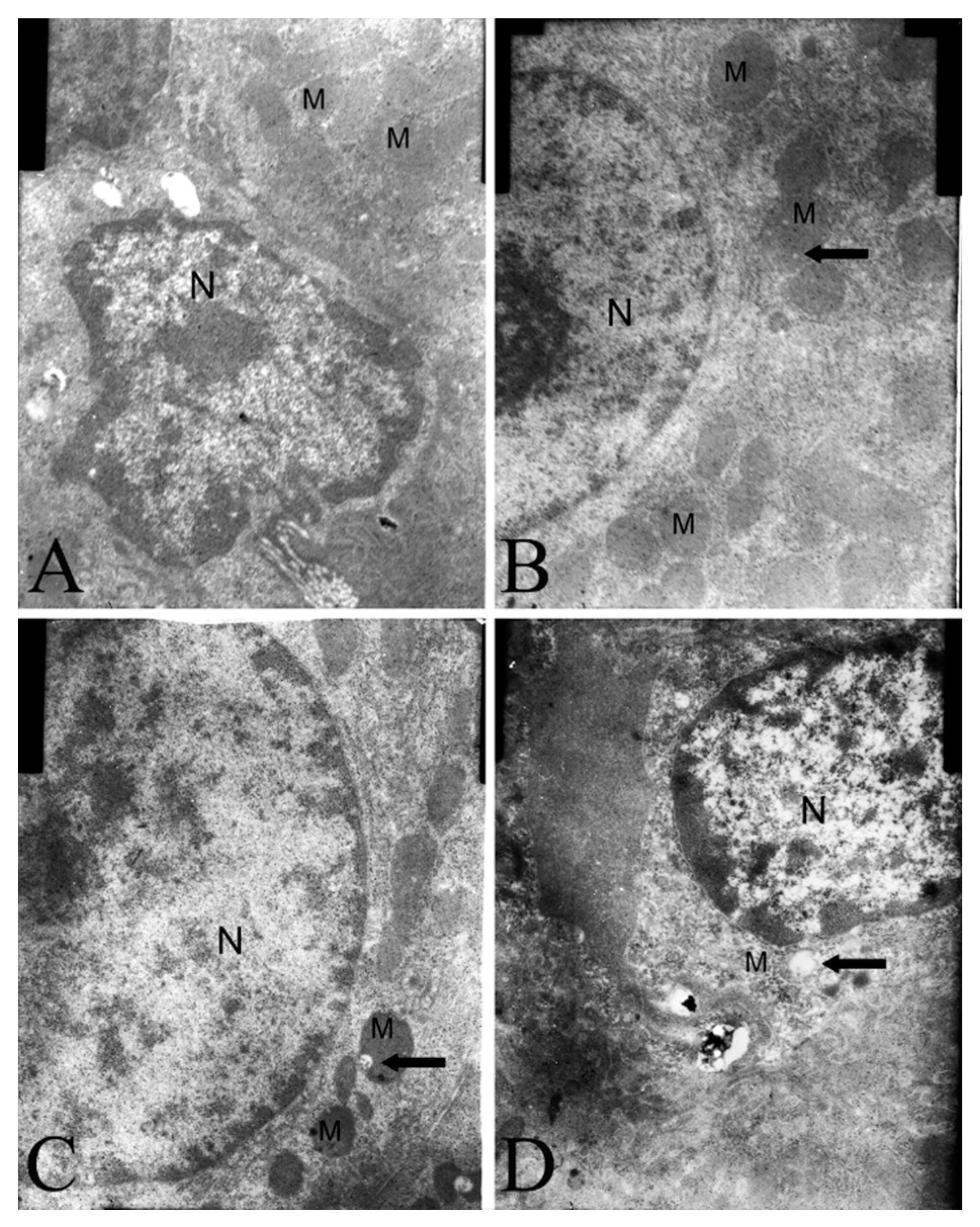
2.5. Effect on Mitochondrial Transmembrane Potential

2.6. Effect of EF on ATP



2.7. Effect on CytC Release
3. Discussion
4. Experimental
4.1. Plant Material and Extract Preparation
4.2. Chemicals
4.3. HPLC Analysis
4.4. Animals
4.5. Experimental Design
4.6. Isolation of Liver Mitochondria
4.7. Assessment of MnSOD, GSH, MDA and ATP
4.8. Assessment of Mitochondrial Permeability Transition
4.9. Electron Microscopy
4.10. Assessment of Mitochondrial Membrane Potential
4.11. Assessment of CytC Release
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- National Pharmacopoeia Committee. Pharmacopoeia of People’s Republic of China, Part 1; The Medicine Science and Technology Press of China: Beijing, China, 2010; pp. 160–161. [Google Scholar]
- Jia, S.; Hu, C. Pharmacological effects of rutaecarpine as a cardiovascular protective agent. Molecules 2010, 15, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.C.; Wu, M.S.; Shen, S.C.; Ko, C.H.; Chen, C.H.; Yang, L.L.; Chen, Y.C. Activation of JNK contributes to evodiamine-induced apoptosis and G2/M arrest in human colorectal carcinoma cells: A structure-activity study of evodiamine. PLoS One 2014, 9, e99729. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, M.; Matsubara, T.; Suzuki, H. Screening of natural compounds for inhibitory activity on colon cancer cell migration. Biol. Pharm. Bull. 2001, 24, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.L.; Li, G.; Moon, D.C.; Lee, C.S.; Woo, M.H.; Lee, E.S.; Jahng, Y.; Chang, H.W.; Lee, S.H.; Son, J.K. Cytotoxicity and DNA topoisomerase inhibitory activity of constituents isolated from the fruits of Evodia officinalis. Arch. Pharm. Res. 2006, 29, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Q.; Xu, Y.; Liu, C.; Wang, X.; He, X.; Zhu, N.; Liu, J.; Wu, Y.; Li, Y.; et al. Rutaecarpine suppresses atherosclerosis in ApoE−/− mice through upregulating ABCA1 and SR-BI within RCT. J. Lipid Res. 2014, 55, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.R. Pharmacological effects of rutaecarpine, an alkaloid isolated from Evodia rutaecarpa. Cardiovasc. Drug Rev. 1999, 17, 237–245. [Google Scholar] [CrossRef]
- Tan, M.X.; Liu, Y.C.; Luo, X.J.; Li, D.Q. Studies on the antioxidant activities of total alkaloids from the fruits of Evodia rutaecarpa (Juss.) Benth. Adv. Mater. Res. 2012, 396, 52–55. [Google Scholar] [CrossRef]
- Cai, X.Y.; Meng, N.; Yang, B. Analysis of one poisoning case caused by excessive Evodiae fructus. Beijing Tradit. Chin. Med. 2006, 25, 171–172. [Google Scholar]
- Cohen, S.M.; Heywood, E.; Pillai, A.; Ahn, J. Hepatotoxicity associated with the use of White Flood, a nutritional supplement. Pract. Gastroenterol. 2012, 10, 45–48. [Google Scholar]
- Teschke, R. Traditional Chinese Medicine induced liver injury. J. Clin. Translat. Hepatol. 2014, 2, 80–94. [Google Scholar] [CrossRef]
- Teschke, R.; Wolff, A.; Frenzel, C.; Schulze, J. Letter: Herbal hepatotoxicity—An update on traditional Chinese medicine preparations; authors’s reply. Aliment. Pharmacol. Ther. 2014, 40, 738–740. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W. Toxicological assessment on safety of water and 70% ethanolic extracts of nearly ripe fruit of Evodia rutaecarpa. Zhongguo Zhong Yao Za Zhi 2008, 33, 1317–1321. [Google Scholar] [PubMed]
- Zhou, Q.; Zhang, Q.; Jin, R.M. Time-effect and dose-effect of Evodia rutaecarpa on hepatotoxicity in mice. Chin. J. Exp. Tradit. Med. Formulae 2011, 17, 232–235. [Google Scholar]
- Huang, W.; Sun, R. Study on chronic toxicity of water extraction components from Evodiafructus in Rats. Chin. J. Exp. Tradit. Med. Formulae 2013, 19, 269–273. [Google Scholar]
- Huang, W.; Li, X.; Sun, R. “Dose-time-toxicity” relationship study on hepatotoxicity caused by multiple dose water extraction components of Evodiae Fructus to mice. Zhongguo Zhong Yao Za Zhi 2012, 37, 2223–2227. [Google Scholar] [PubMed]
- Kim, D.; Lee, Y.H.; Park, S.H.; Lee, M.J.; Kim, M.J.; Jang, H.S.; Lee, J.M.; Lee, H.Y.; Han, B.S.; Son, W.C.; et al. Subchronic oral toxicity of evodia fruit powder in rats. J. Ethnopharmacol. 2014, 151, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Li, L. Research progress on mechanism of drug-induced hepatotoxicity. Fudan Univ. J. Med. Sci. 2007, 34, 313–316. [Google Scholar]
- Deng, X.; Luyendyk, J.P.; Ganey, P.E.; Roth, R.A. Inflammatory stress and idiosyncratic hepatotoxicity: Hints from Animal Models. Pharmacol. Rev. 2009, 61, 262–282. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; Gores, G.J.; Cederbaum, A.I.; Hinson, J.A.; Pessayre, D.; Lemasters, J.J. Mechanisms of hepatotoxicity. Toxicol. Sci. 2002, 65, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; Williams, C.D.; Ramachandran, A.; Bajt, M.L. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 2012, 32, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Masubuchi, Y.; Kano, S.; Horie, T. Mitochondrial permeability transition as a potential determinant of hepatotoxicity of antidiabetic thialozidinediones. Toxicology 2006, 222, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Masubuchi, Y.; Suda, C.; Horie, T. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J. Hepatol. 2005, 42, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Labbe, G.; Pessayre, D.; Fromenty, B. Drug-induced liver injury through mitochondrial dysfunction: Mechanisms and detection during preclinical safety studies. Fundam. Clin. Pharmacol. 2008, 22, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Pessayre, D.; Mansouri, A.; Berson, A.; Fromenty, B. Mitochondrial involvement in drug-induced liver injury. Handb. Exp. Pharmacol. 2010, 196, 311–365. [Google Scholar] [PubMed]
- Teschke, R.; Wolff, A.; Frenzel, C.; Schulze, J. Review article: Herbal hepatotoxicity—An update on traditional Chinese medicine preparations. Aliment. Pharmacol. Ther. 2014, 40, 32–50. [Google Scholar] [CrossRef]
- Lee, W.M. Drug-induced hepatotoxicity. N. Engl. J. Med. 2003, 349, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; McGill, M.R.; Ramachandran, A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: Lessons learned from acetaminophen hepatotoxicity. Drug Metab. Rev. 2012, 44, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Qu, S.; Shi, Q.; He, D.; Jin, X. Evodiamine induces apoptosis and enhances TRAIL-induced apoptosis in human bladder cancer cells through mTOR/S6K1-mediated downregulation of Mcl-1. Int. J. Mol. Sci. 2014, 15, 3154–3171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, Q.; Jin, R.M.; Yao, G.T.; Chen, X.M. Preliminary study on hepatotoxicity and nephrotoxicity induced by rutaecarpine. Chin. J. Exp. Tradit. Med. Formulae 2011, 17, 221–225. [Google Scholar]
- Zhou, Q.; Jin, R.M.; Yao, G.T. Preliminary study on nephrocytes toxicity induced by four traditional Chinese medicine monomers in evodia rutaecarpa. Chin. J. Pharmacovigil. 2013, 10, 1–5. [Google Scholar]
- Negre-Salvayre, A.; Auge, N.; Ayala, V.; Basaga, H.; Boada, J.; Brenke, R.; Chapple, S.; Cohen, G.; Feher, J.; Grune, T.; et al. Pathological aspects of lipid peroxidation. Free Radic. Res. 2010, 44, 1125–1171. [Google Scholar] [CrossRef] [PubMed]
- Watson, W.H.; Yang, X.; Choi, Y.E.; Jones, D.P.; Kehrer, J.P. Thioredoxin and its role in toxicology. Toxicol. Sci. 2004, 78, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Kasznicki, J.; Kosmalski, M.; Sliwinska, A.; Mrowicka, M.; Stanczyk, M.; Majsterek, I.; Drzewoski, J. Evaluation of oxidative stress markers in pathogenesis of diabetic neuropathy. Mol. Biol. Rep. 2012, 39, 8669–8678. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Lebofsky, M.; Baines, C. P.; Lemasters, J.J.; Jaeschke, H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic. Res. 2011, 45, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Zamzami, N.; Hirsch, T.; Dallaporta, B.; Petit, P.X.; Kroemer, G. Mitochondrial implication in accidental and programmed cell death: Apoptosis and necrosis. J. Bioenerg. Biomembr. 1997, 29, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Trost, L.C.; Lemasters, J.J. The mitochondrial permeability transition: a new pathophysiological mechanism for Reye’s syndrome and toxic liver injury. J. Pharmacol. Exp. Ther. 1996, 278, 1000–1005. [Google Scholar] [PubMed]
- Masubuchi, Y.; Nakayama, S.; Horie, T. Role of mitochondrial permeability transition in diclofenac-induced hepatocyte injury in rats. Hepatology 2002, 35, 544–551. [Google Scholar] [CrossRef]
- Haasio, K.; Lounatmaa, K.; Sukura, A. Entacapone does not induce conformational changes in liver mitochondria or skeletal muscle in vivo. Exp. Toxic. Pathol. 2002, 54, 9–14. [Google Scholar] [CrossRef]
- Haasio, K.; Koponen, A.; Penttilä, K.E.; Nissinen, E. Effects of entacapone and tolcapone on mitochondrial membrane potential. Eur. J. Pharmacol. 2002, 453, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Reed, J.C. Mitochondria and apoptosis. Science 1998, 281, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.A.; Song, B.J. Critical role of c-Jun N-terminal protein kinase activation in troglitazone-induced apoptosis of human HepG2 hepatoma cells. Mol. Pharmacol. 2003, 63, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, B.K.; Liu, Z.X.; Han, D.; Hanawa, N.; Gaarde, W.A.; Kaplowitz, N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology 2006, 131, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the plant and extract are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Q.; Wei, J.; Zhao, W.; Shi, S.; Zhang, Y.; Wei, R.; Zhang, Y.; Li, W.; Wang, Q. Toxicity of Evodiae fructus on Rat Liver Mitochondria: The Role of Oxidative Stress and Mitochondrial Permeability Transition. Molecules 2014, 19, 21168-21182. https://doi.org/10.3390/molecules191221168
Cai Q, Wei J, Zhao W, Shi S, Zhang Y, Wei R, Zhang Y, Li W, Wang Q. Toxicity of Evodiae fructus on Rat Liver Mitochondria: The Role of Oxidative Stress and Mitochondrial Permeability Transition. Molecules. 2014; 19(12):21168-21182. https://doi.org/10.3390/molecules191221168
Chicago/Turabian StyleCai, Qingyan, Jingjing Wei, Wei Zhao, Si Shi, Yu Zhang, Renrong Wei, Yue Zhang, Weirong Li, and Qi Wang. 2014. "Toxicity of Evodiae fructus on Rat Liver Mitochondria: The Role of Oxidative Stress and Mitochondrial Permeability Transition" Molecules 19, no. 12: 21168-21182. https://doi.org/10.3390/molecules191221168
APA StyleCai, Q., Wei, J., Zhao, W., Shi, S., Zhang, Y., Wei, R., Zhang, Y., Li, W., & Wang, Q. (2014). Toxicity of Evodiae fructus on Rat Liver Mitochondria: The Role of Oxidative Stress and Mitochondrial Permeability Transition. Molecules, 19(12), 21168-21182. https://doi.org/10.3390/molecules191221168




