Synthesis, Biological Evaluation and Structure-Activity Relationships of New Quinoxaline Derivatives as Anti-Plasmodium falciparum Agents
Abstract
:1. Introduction

2. Results and Discussion
2.1. Chemistry


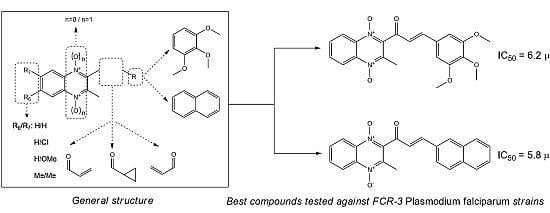
2.2. Biological Results
| Compound | R6 | R7 | n | R | IC50 (μM) a | SD b |
|---|---|---|---|---|---|---|
| 1a | H | H | 1 | 3,4,5-trimethoxyphenyl | 6.2 | 1.7 |
| 1b | H | Cl | 1 | 3,4,5-trimethoxyphenyl | 27.3 | 3.6 |
| 1c | H | OMe | 1 | 3,4,5-trimethoxyphenyl | 31.1 | 20.4 |
| 1d | Me | Me | 1 | 3,4,5-trimethoxyphenyl | 24.4 | 14.0 |
| 2a | H | H | 1 | naphthyl | 5.8 | 0.8 |
| 3a | H | H | 0 | 3,4,5-trimethoxyphenyl | 21.5 | 5.3 |
| 3c | H | OMe | 0 | 3,4,5-trimethoxyphenyl | 26.4 | 0.3 |
| CQ c | - | - | - | - | 0.173 | 0.003 |
| Compound | R6 | R7 | n | R | IC50 (μM) a | SD b |
|---|---|---|---|---|---|---|
| 4a | H | H | 1 | 3,4,5-trimethoxyphenyl | 56.4 | 6.4 |
| 4b | H | Cl | 1 | 3,4,5-trimethoxyphenyl | 20.1 | 6.9 |
| 4c | H | OMe | 1 | 3,4,5-trimethoxyphenyl | NA d | - |
| 4d | Me | Me | 1 | 3,4,5-trimethoxyphenyl | NA | - |
| 5a | H | H | 1 | naphthyl | 62.1 | 20.0 |
| 6a | H | H | 0 | 3,4,5-trimethoxyphenyl | NA | - |
| 6c | H | OMe | 0 | 3,4,5-trimethoxyphenyl | NA | - |
| CQ c | - | - | - | - | 0.173 | 0.003 |
| Compound | R6 | R7 | IC50 (μM) a | SD b |
|---|---|---|---|---|
| 7a | H | H | 29.0 | 3.5 |
| 7b | H | Cl | 24.2 | 0.8 |
| 7c | H | OMe | 34.3 | 2.0 |
| 7d | Me | Me | 24.5 | 4.7 |
| CQ c | - | - | 0.173 | 0.003 |
3. Experimental
3.1. General Information
3.2. General Procedure of Synthesis
3.2.1. Synthesis of 3-Methyl-2-[3-(naphth-2-yl-prop-2-enoyl)] quinoxaline 1,4-di-N-oxide Derivatives (Series 2)
3.2.2. Synthesis of 3-Methyl-2-[2-(3,4,5-trimethoxyphenyl)cyclopropanecarbonyl] quinoxaline 1,4-di-N-oxide Derivatives (Series 4), 3-Methyl-2-[2-(2-naphthyl)cyclopropanecarbonyl] quinoxaline 1,4-di-N-oxide Derivatives (Series 5) and 3-Methyl-2-[2-(3,4,5-trimethoxyphenyl)cyclopropanecarbonyl] quinoxaline Derivatives (Series 6)
3.3. In Vitro Antiplasmodial Drug Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2012. Available online: http://www.who.int/ malaria/publications/world_malaria_report_2012/en/ (accessed on 7 March 2013).
- Snow, R.W.; Guerra, C.A.; Noor, A.M.; Myint, H.Y.; Hay, S.I. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 2005, 434, 214–217. [Google Scholar] [CrossRef]
- Dondorp, A.M.; Nosten, F.; Yi, P.; Das, D.; Phyo, A.P.; Tarning, J.; Lwin, K.M.; Ariey, F.; Hanpithakpong, W.; Lee, S.J.; et al. Artemisinin resistance in Plasmodium falciparum malaria. N. Eng. J. Med. 2009, 361, 455–467. [Google Scholar] [CrossRef]
- White, N.J. Drug resistance in malaria. Br. Med. Bull. 1998, 54, 703–715. [Google Scholar] [CrossRef]
- Rathod, P.K.; McErlean, T.; Lee, P.-C. Variations in frequencies of drug resistance in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 1997, 94, 9389–9393. [Google Scholar] [CrossRef]
- Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A. Synthesis and antimycobacterial activity of new quinoxaline-2-carboxamide 1,4-di-N-oxide derivatives. Bioorg. Med. Chem. 2003, 11, 2149–2156. [Google Scholar] [CrossRef]
- Jaso, A.; Zarranz, B.; Aldana, I.; Monge, A. Synthesis of new 2-acetyl and 2-benzoyl quinoxaline 1,4-di-N-oxide derivatives as anti-Mycobacterium tuberculosis agents. Eur. J. Med. Chem. 2003, 38, 791–800. [Google Scholar]
- Jaso, A.; Zarranz, B.; Aldana, I.; Monge, A. Synthesis of new quinoxaline-2-carboxylate 1,4-dioxide derivatives as anti-Mycobacterium tuberculosis agents. J. Med. Chem. 2005, 48, 2019–2025. [Google Scholar] [CrossRef]
- Vicente, E.; Villar, R.; Burguete, A.; Solano, B.; Pérez-Silanes, S.; Aldana, I.; Maddry, J.A.; Lenaerts, A.J.; Franzblau, S.G.; Cho, S.; et al. Efficacy of quinoxaline-2-carboxylate 1,4-di-N-oxide derivatives in experimental tuberculosis. Antimicrob. Agents Chemother. 2008, 52, 3321–3326. [Google Scholar] [CrossRef]
- Vicente, E.; Pérez-Silanes, S.; Lima, L.M.; Ancizu, S.; Burguete, A.; Solano, B.; Villar, R.; Aldana, I.; Monge, A. Selective activity against Mycobacterium tuberculosis of new quinoxaline 1,4-di-N-oxides. Bioorg. Med. Chem. 2009, 17, 385–389. [Google Scholar]
- Ancizu, S.; Moreno, E.; Solano, B.; Villar, R.; Burguete, A.; Torres, E.; Pérez-Silanes, S.; Aldana, I.; Monge, A. New 3-methylquinoxaline-2-carboxamide 1,4-di-N-oxide derivatives as anti-Mycobacterium tuberculosis agents. Bioorg. Med. Chem. 2010, 18, 2713–2719. [Google Scholar] [CrossRef]
- Moreno, E.; Ancizu, S.; Pérez-Silanes, S.; Torres, E.; Aldana, I.; Monge, A. Synthesis and antimycobacterial activity of new quinoxaline-2-carboxamide 1,4-di-N-oxide derivatives. Eur. J. Med. Chem. 2010, 45, 4418–4426. [Google Scholar] [CrossRef]
- Torres, E.; Moreno, E.; Ancizu, S.; Barea, C.; Galiano, S.; Aldana, I.; Monge, A.; Pérez-Silanes, S. New 1,4-di-N-oxide-quinoxaline-2-ylmethylene isonicotinic acid hydrazide derivatives as anti-Mycobacterium tuberculosis agents. Bioorg. Med. Chem. Lett. 2011, 21, 3699–3703. [Google Scholar] [CrossRef]
- Aguirre, G.; Cerecetto, H.; di Maio, R.; González, M.; Montoya Alfaro, M.E.; Jaso, A.; Zarranz, B.; Ortega, M.A.; Aldana, I.; Monge-Vega, A. Quinoxaline N,N'-dioxide derivatives and related compounds as growth inhibitors of Trypanosoma cruzi. Structure-activity relationships. Bioorg. Med. Chem. Lett. 2004, 14, 3835–3839. [Google Scholar] [CrossRef]
- Benitez, D.; Cabrera, M.; Hernández, P.; Boiani, L.; Lavaggi, M.L.; di Maio, R.; Yaluff, G.; Serna, E.; Torres, S.; Ferreira, M.E.; et al. 3-Trifluoromethylquinoxaline N,N’-dioxides as anti-trypanosomatid agents. Identification of optimal anti-T. cruzi agents and mechanism of action studies. J. Med. Chem. 2011, 54, 3624–3636. [Google Scholar] [CrossRef]
- Barea, C.; Pabón, A.; Castillo, D.; Zimic, M.; Quiliano, M.; Galiano, S.; Pérez-Silanes, S.; Monge, A.; Deharo, E.; Aldana, I. New salicylamide and sulfonamide derivatives of quinoxaline 1,4-di-N-oxide with antileishmanial and antimalarial activities. Bioorg. Med. Chem. Lett. 2011, 21, 4498–4502. [Google Scholar] [CrossRef]
- Barea, C.; Pabón, A.; Galiano, S.; Pérez-Silanes, S.; Gonzalez, G.; Deyssard, C.; Monge, A.; Deharo, E.; Aldana, I. Antiplasmodial and leishmanicidal activities of 2-cyano-3-(4-phenylpiperazine-1-carboxamido) quinoxaline 1,4-dioxide derivatives. Molecules 2012, 17, 9451–9461. [Google Scholar] [CrossRef]
- Marin, A.; Lima, L.M.; Solano, B.; Vicente, E.; Pérez Silanes, S.; Maurel, S.; Sauvain, M.; Aldana, I.; Monge, A.; Deharo, E. Antiplasmodial structure-activity relationship of 3-trifluoromethyl-2-arylcarbonylquinoxaline 1,4-di-N-oxide derivatives. Exp. Parasitol. 2008, 118, 25–31. [Google Scholar] [CrossRef]
- Vicente, E.; Lima, L.M.; Bongard, E.; Charnaud, S.; Villar, R.; Solano, B.; Burguete, A.; Perez-Silanes, S.; Aldana, I.; Vivas, L.; et al. Synthesis and structure-activity relationship of 3-phenylquinoxaline 1,4-di-N-oxide derivatives as antimalarial agents. Eur. J. Med. Chem. 2008, 43, 1903–1910. [Google Scholar] [CrossRef]
- Vicente, E.; Charnaud, S.; Bongard, E.; Villar, R.; Burguete, A.; Solano, B.; Ancizu, S.; Pérez-Silanes, S.; Aldana, I.; Vivas, L.; et al. Synthesis and antiplasmodial activity of 3-furyl and 3-thienylquinoxaline-2-carbonitrile 1,4-di-N-oxide derivatives. Molecules 2008, 13, 69–77. [Google Scholar]
- Maichrowski, J.; Gjikaj, M.; Hübner, E.G.; Bergmann, B.; Müller, I.B.; Kaufmann, D.E. Efficient synthesis of quinoxaline derivatives by selective modification of 3-chloro-6-fluoroquinoxalin-2(1H)-one 4-oxide. Eur. J. Org. Chem. 2013, 11, 2091–2105. [Google Scholar]
- Barea, C.; Pabón, A.; Pérez-Silanes, S.; Galiano, S.; González, G.; Monge, A.; Deharo, E.; Aldana, I. New amide derivatives of quinoxaline 1,4-di-N-oxide with leishmanicidal and antiplasmodial activities. Molecules 2013, 18, 4718–4727. [Google Scholar] [CrossRef]
- Królikiewicz, M.; Wróbel, Z. Simple synthesis of quinoxalin-2(1H)-one N-oxides from N-aryl-2-nitrosoanilines and alkylated cyanoacetic esters. J. Heterocycl. Chem. 2014, 51, 123–126. [Google Scholar] [CrossRef]
- Monge, A.; Palop, J.A.; López de Ceráin, A.; Senador, V.; Martínez-Crespo, F.J.; Sainz, Y.; Narro, S.; García, E.; de Miguel, C.; González, M.; et al. Hypoxia-selective agents derived from quinoxaline 1,4-di-N-oxides. J. Med. Chem. 1995, 38, 1786–1792. [Google Scholar] [CrossRef]
- Ortega, M.A.; Morancho, M.J.; Martínez-Crespo, F.J.; Sainz, Y.; Montoya, M.E.; López de Ceráin, A.; Monge, A. New quinoxalinecarbonitrile 1,4-di-N-oxide derivatives as hypoxic-cytotoxic agents. Eur. J. Med. Chem. 2000, 35, 21–30. [Google Scholar]
- Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A. Synthesis and anticancer activity evaluation of new 2-alkylcarbonyl and 2-benzoyl-3-trifluoromethyl-quinoxaline 1,4-di-N-oxide derivatives. Bioorg. Med. Chem. 2004, 12, 3711–3721. [Google Scholar] [CrossRef]
- Solano, B.; Junnotula, V.; Marín, A.; Villar, R.; Burguete, A.; Vicente, E.; Pérez-Silanes, S.; Aldana, I.; Monge, A.; Dutta, S.; et al. Synthesis and biological evaluation of new 2-arylcarbonyl-3-trifluoromethylquinoxaline 1,4-di-N-oxide derivatives and their reduced analogues. J. Med. Chem. 2007, 50, 5485–5492. [Google Scholar] [CrossRef]
- Burguete, A.; Pontiki, E.; Hadjipavlou-Litina, D.; Ancizu, S.; Villar, R.; Solano, B.; Moreno, E.; Torres, E.; Pérez, S.; Aldana, I.; et al. Synthesis and biological evaluation of new quinoxaline derivatives as antioxidant and anti-inflammatory agents. Chem. Biol. Drug Des. 2011, 77, 255–267. [Google Scholar]
- Montoya, M.E.; Sainz, Y.; Ortega, M.A.; Lopez de Cerain, A.; Monge, A. Synthesis and antituberculosis activity of some new 2-quinoxalinecarbonitriles. Il Farmaco 1998, 53, 570–573. [Google Scholar] [CrossRef]
- Dimmock, J.R.; Elias, D.W.; Beazely, M.A.; Kandepu, N.M. Bioactivities of chalcones. Curr. Med. Chem. 1999, 6, 1125–1149. [Google Scholar]
- Herencia, F.; Ferrándiz, M.L.; Ubeda, A.; Domínguez, J.N.; Charris, J.E.; Lobo, G.M.; Alcaraz, M.J. Synthesis and anti-inflammatory activity of chalcone derivatives. Bioorg. Med. Chem. Lett. 1998, 8, 1169–1174. [Google Scholar]
- Ducki, S.; Forrest, R.; Hadfield, J.A.; Kendall, A.; Lawrence, N.J.; McGown, A.T.; Rennison, D. Potent antimitotic and cell growth inhibitory properties of substituted chalcones. Bioorg. Med. Chem. Lett. 1998, 8, 1051–1056. [Google Scholar]
- Lin, Y.-M.; Zhou, Y.; Flavin, M.T.; Zhou, L.-M.; Nie, W.; Chen, F.-C. Chalcones and flavonoids as anti-tuberculosis agents. Bioorg. Med. Chem. 2002, 10, 2795–2802. [Google Scholar]
- Hans, R.H.; Guantai, E.M.; Lategan, C.; Smith, P.J.; Wan, B.; Franzblau, S.G.; Gut, J.; Rosenthal, P.J.; Chibale, K. Synthesis, antimalarial and antitubercular activity of acetylenic chalcones. Bioorg. Med. Chem. Lett. 2010, 20, 942–944. [Google Scholar]
- Smit, F.J.; N’Da, D.D. Synthesis, in vitro antimalarial activity and cytotoxicity of novel 4-aminoquinolinyl-chalcone amides. Bioorg. Med. Chem. 2014, 22, 1128–1138. [Google Scholar] [CrossRef]
- Tomar, V.; Bhattacharjee, G.; Kamaluddin; Rajakumar, S.; Srivastava, K.; Puri, S.K. Synthesis of new chalcone derivatives containing acridinyl moiety with potencial antimalarial activity. Eur. J. Med. Chem. 2010, 45, 745–751. [Google Scholar]
- Sashidhara, K.V.; Kumar, M.; Modukuri, R.K.; Srivastava, R.K.; Soni, A.; Srivastava, K.; Singh, S.V.; Saxena, J.K.; Gauniyal, H.M.; Puri, S.K. Antiplasmodial activity of novel keto-enamine chalcone-chloroquine based hybrid pharmacophores. Bioorg. Med. Chem. 2012, 20, 2971–2981. [Google Scholar]
- Guantai, E.M.; Ncokazi, K.; Egan, T.J.; Gut, J.; Rosenthal, P.J.; Smith, P.J.; Chibale, K. Design, synthesis and in vitro antimalarial evaluation of triazole-linked chalcone and dienone hybrid compounds. Bioorg. Med. Chem. 2010, 18, 8243–8256. [Google Scholar] [CrossRef]
- Larsen, M.; Kromann, H.; Kharazni, A.; Nielsen, S.F. Conformationally restricted anti-plasmodial chalcones. Bioorg. Med. Chem. Lett. 2005, 15, 4858–4861. [Google Scholar]
- Burguete, A.; Estevez, Y.; Castillo, D.; González, G.; Villar, R.; Solano, B.; Vicente, E.; Pérez Silanes, S.; Aldana, I.; Monge, A.; et al. Anti-leishmanial and structure-activity relationship of ring substituted 3-phenyl-1-(1,4-di-N-oxide quinoxalin-2-yl)-2-propen-1-one derivatives. Mem. Inst. Oswaldo Cruz 2008, 103, 778–780. [Google Scholar] [CrossRef]
- Estevez, Y.; Quiliano, M.; Burguete, A.; Cabanillas, B.; Zimic, M.; Málaga, E.; Verástegui, M.; Pérez-Silanes, S.; Aldana, I.; Monge, A.; et al. Trypanocidal properties, structure-activity relationship and computational studies of quinoxaline 1,4-di-N-oxide derivatives. Exp. Parasitol. 2011, 127, 745–751. [Google Scholar] [CrossRef]
- Das, U.; Pati, H.N.; Panda, A.K.; de Clercq, E.; Balzarini, J.; Molnár, J.; Baráth, Z.; Ocsovszki, I.; Kawase, M.; Zhou, L.; et al. 2-(3-Aryl-2-propenoyl)-3-methylquinoxaline-1,4-dioxides: a novel cluster of tumor-specific cytotoxins which reverse multidrug resistance. Bioorg. Med. Chem. 2009, 17, 3909–3915. [Google Scholar] [CrossRef]
- Burguete, A.; Pontiki, E.; Hadjipavlou-Litina, D.; Villar, R.; Vicente, E.; Solano, B.; Ancizu, S.; Pérez-Silanes, S.; Aldana, I.; Monge, A. Synthesis and anti-inflammatory/antioxidant activities of some new ring substituted 3-phenyl-1-(1,4-di-N-oxide quinoxalin-2-yl)-2-propen-1-one derivatives and of their 4,5-dihydro-(1H)-pyrazole analogues. Bioorg. Med. Chem. Lett. 2007, 17, 6439–6443. [Google Scholar] [CrossRef]
- Mufarrij, N.A.; Haddadin, M.J.; Issidorides, C.H.; McFarland, J.W.; Johnston, J.D. The reactions of benzofurazan 1-oxides with enamines. J. Chem. Soc. Perkin Trans. 1 1972, 7, 965–967. [Google Scholar]
- Haddadin, M.J.; Issidorides, C.H. Application of benzofurazan oxide to the synthesis of heteroaromatic N-oxides. Heterocycles 1976, 4, 767–816. [Google Scholar] [CrossRef]
- Stumm, G.; Niclas, H.J. An improved and efficient synthesis of quinoxalinecarboxamide 1,4-dioxides from benzofuroxan and acetoacetamides in the presence of calcium salts. J. Prakt. Chem. 1989, 331, 736–744. [Google Scholar] [CrossRef]
- Burguete, A. Design, Synthesis and Biological Evaluation of New Quinoxaline and Quinoxaline 1,4-Di-N-oxide Derivatives with Application in Different Therapeutic Areas. M.S. Thesis, Universidad de Navarra, Pamplona, Spain, 2010. [Google Scholar]
- Cheeseman, G.W.H.; Cookson, R.F. Chemistry of Heterocyclic Compounds: Condensed Pyrazines; John Wiley and Sons: New York, NY, USA, 1979; Volume 35. [Google Scholar]
- Nielsen, S.F.; Boesen, T.; Larsen, M.; Schønning, K.; Kromann, H. Antibacterial chalcones-bioisosteric replacement of the 4'-hydroxy group. Bioorg. Med. Chem. 2004, 12, 3047–3054. [Google Scholar] [CrossRef]
- Abd El-Halim, M.S.; El-Ahl, A.S.; Etman, H.A.; Ali, M.M.; Fouda, A.; Fadda, A.A. A new route for the synthesis of phenazine di-N-oxides. Mon. Chem. 1995, 126, 1217–1223. [Google Scholar]
- Ajay, A.; Singh, V.; Singh, S.; Pandey, S.; Gunjan, S.; Dubey, D.; Sinha, S.K.; Singh, B.N.; Chaturvedi, V.; Tripathi, R.; et al. Synthesis and bio-evaluation of alkylaminoaryl phenyl cyclopropyl methanones as antitubercular and antimalarial agents. Bioorg. Med. Chem. 2010, 18, 8289–8301. [Google Scholar] [CrossRef]
- Corey, E.J.; Chaykovsky, M. Dimethylsulfoxonium methylide. J. Am. Chem. Soc. 1962, 84, 867–868. [Google Scholar] [CrossRef]
- Gololobov, Y.G.; Nesmeyanov, A.N.; Lysenko, V.P.; Boldeskul, I.E. Twenty-five years of dimethylsulfoxonium methylide (Corey’s reagent). Tetrahedron 1987, 43, 2609–2651. [Google Scholar] [CrossRef]
- Corey, E.J.; Chaykovsky, M. Dimethyloxosulfonium methylide ((CH3)2SOCH2) and dimethylsulfonium methylide ((CH3)2SCH2). Formation and application to organic synthesis. J. Am. Chem. Soc. 1965, 87, 1353–1364. [Google Scholar] [CrossRef]
- Hartikka, A.; Arvidsson, P.I. Tetrazolic acid functionalized dihydroindol: rational design of a highly selective cyclopropanation organocatalyst. J. Org. Chem. 2007, 72, 5874–5877. [Google Scholar] [CrossRef]
- Paxton, R.J.; Taylor, R.J.K. Improved dimethylsulfoxonium methylide cyclopropanation procedures, including a tandem oxidation variant. Synlett 2007, 4, 633–637. [Google Scholar]
- Monge, A.; Gil, M.J.; Gastelurrutia, M.A.; Pascual, M. Potential bactericides. I. Synthesis and bactericidal activity of 1,4-dioxides of 2-acyl and 2-aryl quinoxolinethylenes and pyrazole derivatives. An. Real Acad. Farm. 1982, 48, 533–542. [Google Scholar]
- Zarranz, B.; Jaso, A.; Moreira Lima, L.; Aldana, I.; Monge, A.; Maurel, S.; Sauvain, M. Antiplasmodial activity of 3-trifluoromethyl-2-carbonylquinoxaline di-N-oxide derivatives. Braz. J. Pharm. Sci. 2006, 42, 357–361. [Google Scholar] [CrossRef]
- Desjardins, R.E.; Canfield, C.J.; Haynes, J.D.; Chulay, J.D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 1979, 16, 710–718. [Google Scholar] [CrossRef]
- Sereno, D.; Lemesre, J.L. Use of an enzymatic micromethod to quantify amastigote stage of Leishmania amazonensis in vitro. Parasitol. Res. 1997, 83, 401–403. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the synthesized compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gil, A.; Pabón, A.; Galiano, S.; Burguete, A.; Pérez-Silanes, S.; Deharo, E.; Monge, A.; Aldana, I. Synthesis, Biological Evaluation and Structure-Activity Relationships of New Quinoxaline Derivatives as Anti-Plasmodium falciparum Agents. Molecules 2014, 19, 2166-2180. https://doi.org/10.3390/molecules19022166
Gil A, Pabón A, Galiano S, Burguete A, Pérez-Silanes S, Deharo E, Monge A, Aldana I. Synthesis, Biological Evaluation and Structure-Activity Relationships of New Quinoxaline Derivatives as Anti-Plasmodium falciparum Agents. Molecules. 2014; 19(2):2166-2180. https://doi.org/10.3390/molecules19022166
Chicago/Turabian StyleGil, Ana, Adriana Pabón, Silvia Galiano, Asunción Burguete, Silvia Pérez-Silanes, Eric Deharo, Antonio Monge, and Ignacio Aldana. 2014. "Synthesis, Biological Evaluation and Structure-Activity Relationships of New Quinoxaline Derivatives as Anti-Plasmodium falciparum Agents" Molecules 19, no. 2: 2166-2180. https://doi.org/10.3390/molecules19022166