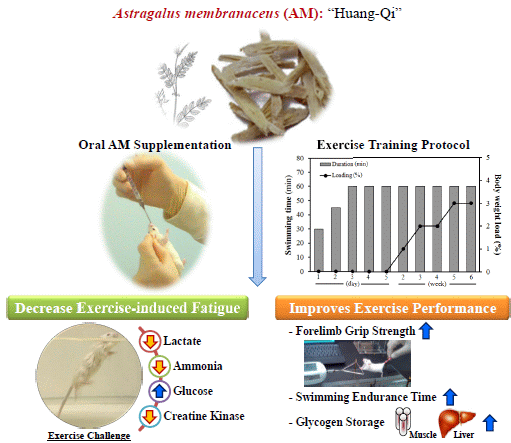
Astragalus membranaceus Improves Exercise Performance and Ameliorates Exercise-Induced Fatigue in Trained Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Body Weight and Other Metabolism-Related Organ Weights
| Characteristic | Vehicle Control | Exercise Control | Ex-AM1 | Ex-AM5 |
|---|---|---|---|---|
| Initial body weight (g) | 25.43 ± 0.24 | 25.47 ± 0.20 | 25.53 ± 0.29 | 25.49 ± 0.28 |
| 1 week body weight (g) | 29.94 ± 0.57 | 29.11 ± 0.40 | 28.76 ± 0.50 | 28.78 ± 0.57 |
| 2 week body weight (g) | 31.54 ± 0.59 | 30.07 ± 0.49 | 31.55 ± 0.50 | 31.41 ± 0.68 |
| 3 week body weight (g) | 32.63 ± 0.48 | 32.43 ± 0.50 | 32.84 ± 0.48 | 33.26 ± 0.85 |
| 4 week body weight (g) | 33.52 ± 0.44 | 33.53 ± 0.41 | 33.52 ± 0.53 | 34.23 ± 0.85 |
| 5 week body weight (g) | 34.50 ± 0.45 | 33.68 ± 0.33 | 34.59 ± 0.56 | 35.11 ± 0.81 |
| Final body weight (g) | 35.59 ± 0.51 | 35.80 ± 0.46 | 36.28 ± 0.44 | 37.18 ± 0.76 |
| Food intake (g/day) | 6.78 ± 0.01 a | 7.61 ± 0.01 d | 7.44 ± 0.03 c | 7.11 ± 0.03 b |
| Food efficiency (%) | 1.49 ± 0.06 | 1.35 ± 0.05 | 1.44 ± 0.05 | 1.64 ± 0.08 |
| Liver (g) | 2.16 ± 0.03 | 2.14 ± 0.03 | 2.05 ± 0.03 | 2.06 ± 0.04 |
| Kidney (g) | 0.61 ± 0.01 | 0.65 ± 0.02 | 0.62 ± 0.02 | 0.63 ± 0.01 |
| Epididymal fat pads (g) | 0.52 ± 0.03 | 0.47 ± 0.02 | 0.42 ± 0.04 | 0.46 ± 0.02 |
| Muscle (g) | 0.36 ± 0.01 | 0.36 ± 0.01 | 0.37 ± 0.01 | 0.37 ± 0.01 |
| Brown adipose tissue (g) | 0.13 ± 0.01 | 0.15 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.01 |
| Relative liver weight (%) | 6.07 ± 0.07 b | 5.97 ± 0.07 b | 5.67 ± 0.07b | 5.54 ± 0.11 a |
| Relative kidney weight (%) | 1.72 ± 0.03 | 1.81 ± 0.04 | 1.71 ± 0.04 | 1.71 ± 0.04 |
| Relative epididymal fat pads weight (%) | 1.45 ± 0.06 | 1.30 ± 0.07 | 1.17 ± 0.12 | 1.23 ± 0.07 |
| Relative muscle weight (%) | 1.01 ± 0.02 | 1.02 ± 0.02 | 1.02 ± 0.02 | 1.00 ± 0.03 |
| Relative brown adipose tissue weight (%) | 0.37 ± 0.01 | 0.42 ± 0.02 | 0.43 ± 0.03 | 0.43 ± 0.03 |
2.2. Effects of AM on Forelimb Grip Strength

2.3. Effect of AM on Exercise Performance in Weight-loaded Swim Test
2.4. Effect of Exercise Training Combined with AM Supplementation on the Serum Levels of Lactate, Ammonia, Glucose and Creatine Kinase (CK) After Acute Exercise Challenge

2.5. Effect of AM Supplementation on Hepatic and Muscle Glycogen Levels
2.6. Effect of AM Supplementation on Biochemical Analyses at the End of the Experiment

| Parameter | Vehicle Control | Exercise Control | Ex-AM1 | Ex-AM5 |
|---|---|---|---|---|
| AST (U/L) | 62.90 ± 3.17 | 68.90 ± 3.78 | 60.70 ± 2.93 | 59.00 ± 1.97 |
| ALT (U/L) | 42.10 ± 2.84 a | 54.30 ± 2.34 b | 39.20 ± 1.79 a | 46.30 ± 1.93 a,b |
| ALP (U/L) | 48.80 ± 3.22 | 63.40 ± 5.80 | 54.80 ± 3.59 | 59.20 ± 3.49 |
| LDH (U/L) | 301.10 ± 19.06 | 273.00 ± 23.09 | 254.70 ± 17.29 | 293.10 ± 15.41 |
| Albumin (g/dL) | 3.56 ± 0.08 | 3.76 ± 0.06 | 3.59 ± 0.05 | 3.73 ± 0.06 |
| TBIL (μg/dL) | 0.19 ± 0.03 | 0.22 ± 0.03 | 0.23 ± 0.03 | 0.22 ± 0.02 |
| TP (g/dL) | 4.73 ± 0.06 | 4.63 ± 0.06 | 4.56 ± 0.05 | 4.58 ± 0.05 |
| BUN (mg/dL) | 23.43 ± 0.77 | 23.07 ± 1.00 | 20.18 ± 0.57 | 23.21 ± 0.46 |
| Creatinine (mg/dL) | 0.13 ± 0.01 | 0.12 ± 0.01 | 0.11 ± 0.00 | 0.12 ± 0.01 |
| UA (mg/dL) | 1.43 ± 0.11 a | 0.83 ± 0.05 b | 1.33 ± 0.07 a | 1.18 ± 0.09 a,b |
| TG (mg/dL) | 228.00 ± 21.03 a | 184.40 ± 20.34 a,b | 126.70 ± 5.74 b | 136.60 ± 10.02 b |
| TC (mg/dL) | 110.60 ± 4.17 | 104.40 ± 4.45 | 112.80 ± 4.68 | 107.90 ± 4.13 |
| Glucose (mg/dL) | 179.80 ± 6.03 | 182.30 ± 6.73 | 181.00 ± 4.40 | 177.20 ± 4.56 |
2.7. Effect of AM Supplementation on Histological Examinations at the End of the Experiment

2.8. Discussion
3. Experimental
3.1. Experiment Design
3.2. Swimming Exercise Training
3.3. Exhaustion Swimming Exercise Test
3.4. Forelimb Grip Strength
3.5. Determination of Blood Biochemical Variables
3.6. Tissue Glycogen Determination
3.7. Histological Staining of Tissues
3.8. Analysis of Astragalus Membranaceus by HPLC/CAD
| A. membranaceus (mg/g) | |
|---|---|
| Astragaloside I | 1.02 |
| Astragaloside II | 0.24 |
| Astragaloside III | BRL |
| Astragaloside IV | 0.195 |
| Total Astragalosides | 1.455 |
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflictts of Interest
References
- Lee, D.Y.; Noh, H.J.; Choi, J.; Lee, K.H.; Lee, M.H.; Lee, J.H.; Hong, Y.; Lee, S.E.; Kim, S.Y.; Kim, G.S. Anti-inflammatory cycloartane-type saponins of Astragalus. membranaceus. Molecules 2013, 18, 3725–3732. [Google Scholar] [CrossRef]
- Ma, X.Q.; Shi, Q.; Duan, J.A.; Dong, T.T.; Tsim, K.W. Chemical analysis of Radix Astragali (Huangqi) in China: A comparison with its adulterants and seasonal variations. J. Agric. Food Chem. 2002, 50, 4861–4866. [Google Scholar] [CrossRef]
- Agyemang, K.; Han, L.; Liu, E.; Zhang, Y.; Wang, T.; Gao, X. Recent advances in Astragalus membranaceus anti-diabetic research: Pharmacological effects of its phytochemical constituents. Evid. Based Complement. Alternat. Med. 2013, 2013, 654643. [Google Scholar]
- Ren, S.; Zhang, H.; Mu, Y.; Sun, M.; Liu, P. Pharmacological effects of Astragaloside IV: A literature review. J. Tradit. Chin. Med. 2013, 33, 413–416. [Google Scholar] [CrossRef]
- Kwon, H.J.; Hwang, J.; Lee, S.K.; Park, Y.D. Astragaloside content in the periderm, cortex, and xylem of Astragalus. membranaceus root. J. Nat. Med. 2013, 67, 850–855. [Google Scholar] [CrossRef]
- Sinclair, S. Chinese herbs: A clinical review of Astragalus, Ligusticum, and Schizandrae. Altern. Med. Rev. 1998, 3, 338–344. [Google Scholar]
- Yang, J.; Wang, H.X.; Zhang, Y.J.; Yang, Y.H.; Lu, M.L.; Zhang, J.; Li, S.T.; Zhang, S.P.; Li, G. Astragaloside IV attenuates inflammatory cytokines by inhibiting TLR4/NF-кB signaling pathway in isoproterenol-induced myocardial hypertrophy. J. Ethnopharmacol. 2013, 150, 1062–1070. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, J.; He, G.; Sun, X.; Huang, X.; Hao, L. Effects of astragaloside IV on action potentials and ionic currents in guinea-pig ventricular myocytes. Biol. Pharm. Bull. 2013, 36, 515–521. [Google Scholar] [CrossRef]
- Xu, X.L.; Chen, X.J.; Ji, H.; Li, P.; Bian, Y.Y.; Yang, D.; Xu, J.D.; Bian, Z.P.; Zhang, J.N. Astragaloside IV improved intracellular calcium handling in hypoxia-reoxygenated cardiomyocytes via the sarcoplasmic reticulum Ca-ATPase. Pharmacology 2008, 81, 325–332. [Google Scholar] [CrossRef]
- Chen, P.; Xie, Y.; Shen, E.; Li, G.G.; Yu, Y.; Zhang, C.B.; Yang, Y.; Zou, Y.; Ge, J.; Chen, R.; et al. Astragaloside IV attenuates myocardial fibrosis by inhibiting TGF-β1 signaling in coxsackievirus B3-induced cardiomyopathy. Eur. J. Pharmacol. 2011, 658, 168–174. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Q.; Lu, L.; Zhao, X.; Gao, X.; Wang, Y. Astragaloside IV stimulates angiogenesis and increases hypoxia-inducible factor-1α accumulation via phosphatidylinositol 3-kinase/Akt pathway. J. Pharmacol. Exp. Ther. 2011, 338, 485–491. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, G.; Li, S.; Li, Z.H.; Lam, C.O.; Hong, S.J.; Kwan, Y.W.; Chan, S.W.; Leung, G.P.; Lee, S.M. Pro-angiogenic activity of astragaloside IV in HUVECs in vitro and zebrafish in vivo. Mol. Med. Rep. 2012, 5, 805–811. [Google Scholar]
- Wang, S.G.; Xu, Y.; Chen, J.D.; Yang, C.H.; Chen, X.H. Astragaloside IV Stimulates angiogenesis and Increases nitric oxide accumulation via JAK2/STAT3 and ERK1/2 pathway. Molecules 2013, 18, 12809–12819. [Google Scholar] [CrossRef]
- Hong, M.J.; Ko, E.B.; Park, S.K.; Chang, M.S. Inhibitory effect of Astragalus membranaceus root on matrix metalloproteinase-1 collagenase expression and procollagen destruction in ultraviolet B-irradiated human dermal fibroblasts by suppressing nuclear factor kappa-B activity. J. Pharm. Pharmacol. 2013, 65, 142–148. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Huang, H.; Gao, W.; Zhuang, C.; Li, B.; Zhou, P.; Kong, D. Anti-hepatitis B virus activities of astragaloside IV isolated from radix Astragali. Biol. Pharm. Bull. 2009, 32, 132–135. [Google Scholar] [CrossRef]
- Lv, L.; Wu, S.Y.; Wang, G.F.; Zhang, J.J.; Pang, J.X.; Liu, Z.Q.; Xu, W.; Wu, S.G.; Rao, J.J. Effect of astragaloside IV on hepatic glucose-regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin. Phytother. Res. 2010, 24, 219–224. [Google Scholar]
- Liu, Q.Y.; Yao, Y.M.; Zhang, S.W.; Sheng, Z.Y. Astragalus polysaccharides regulate T cell-mediated immunity via CD11c(high)CD45RB(low) DCs in vitro. J. Ethnopharmacol. 2011, 136, 457–464. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Tsai, W.J.; Loke, S.H.; Wu, T.S.; Chiou, W.F. Astragalus membranaceus flavonoids (AMF) ameliorate chronic fatigue syndrome induced by food intake restriction plus forced swimming. J. Ethnopharmacol. 2009, 122, 28–34. [Google Scholar] [CrossRef]
- Huang, L.F.; Yao, Y.M.; Li, J.F.; Zhang, S.W.; Li, W.X.; Dong, N.; Yu, Y.; Sheng, Z.Y. The effect of Astragaloside IV on immune function of regulatory T cell mediated by high mobility group box 1 protein in vitro. Fitoterapia 2012, 83, 1514–1522. [Google Scholar] [CrossRef]
- Mehta, R.K.; Agnew, M.J. Influence of mental workload on muscle endurance, fatigue, and recovery during intermittent static work. Eur. J. Appl. Physiol. 2012, 112, 2891–2902. [Google Scholar] [CrossRef]
- Appell, H.J.; Soares, J.M.; Duarte, J.A. Exercise, muscle damage and fatigue. Sports Med. 1992, 13, 108–115. [Google Scholar] [CrossRef]
- Fitts, R.H. Cellular mechanisms of muscle fatigue. Physiol. Rev. 1994, 74, 49–94. [Google Scholar]
- Ament, W.; Verkerke, G.J. Exercise and fatigue. Sports Med. 2009, 39, 389–422. [Google Scholar] [CrossRef]
- Grandjean, E.P. Fatigue. Am. Ind. Hyg. Assoc. J. 1970, 31, 401–411. [Google Scholar] [CrossRef]
- Ream, E.; Richardson, A. Review Fatigue: A concept analysis. Int. J. Nurs. Stud. 1996, 33, 519–529. [Google Scholar] [CrossRef]
- Coombes, J.S.; Rowell, B.; Dodd, S.L.; Demirel, H.A.; Naito, H.; Shanely, R.A.; Powers, S.K. Effects of vitamin E deficiency on fatigue and muscle contractile properties. Eur. J. Appl. Physiol. 2002, 87, 272–277. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. In vitro antioxidant activity and in vivo anti-fatigue effect of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. Food Chem. 2011, 124, 188–194. [Google Scholar] [CrossRef]
- Hsu, C.C.; Ho, M.C.; Lin, L.C.; Su, B.; Hsu, M.C. American ginseng supplementation attenuate creatine kinase level induces by submaximal exercise in humans. World J. Gastroenterol. 2005, 11, 5327–5331. [Google Scholar]
- Kim, S.; Park, S.H.; Lee, H.N.; Park, T. Prunus mume extract ameliorates exercise-induced fatigue in trained rats. J. Med. Food. 2008, 11, 460–468. [Google Scholar] [CrossRef]
- Yeh, T.S.; Chan, K.H.; Hsu, M.C.; Liu, J.F. Supplementation with soybean peptides, taurine, Pueraria isoflavone, and ginseng saponin complex improves endurance exercise capacity in humans. J. Med. Food. 2011, 14, 219–225. [Google Scholar] [CrossRef]
- Huang, C.C.; Hsu, M.C.; Huang, W.C.; Yang, H.R.; Hou, C.C. Triterpenoid-rich extract from Antrodia camphorata improves physical fatigue and exercise performance in mice. Evid. Based Complement. Alternat. Med. 2012, 2012, 364741. [Google Scholar]
- Gibson, H.; Edwards, R.H. Muscular exercise and fatigue. Sports Med. 1985, 2, 120–132. [Google Scholar] [CrossRef]
- Liu, M.; Wu, K.; Mao, X.; Wu, Y.; Ouyang, J. Astragalus polysaccharide improves insulin sensitivity in KKAy mice: regulation of PKB/GLUT4 signaling in skeletal muscle. J. Ethnopharmacol. 2010, 127, 32–37. [Google Scholar] [CrossRef]
- Henriksen, E.J.; Bourey, R.E.; Rodnick, K.J.; Koranyi, L.; Permutt, M.A.; Holloszy, J.O. Glucose transporter protein content and glucose transport capacity in rat skeletal muscles. Am. J. Physiol. 1990, 259, E593–E598. [Google Scholar]
- Fueger, P.T.; Hess, H.S.; Posey, K.A.; Bracy, D.P.; Pencek, R.R.; Charron, M.J.; Wasserman, D.H. Control of exercise-stimulated muscle glucose uptake by GLUT4 is dependent on glucose phosphorylation capacity in the conscious mouse. J. Biol. Chem. 2004, 279, 50956–50961. [Google Scholar]
- Warren, G.L.; Ingalls, C.P.; Lowe, D.A.; Armstrong, R.B. Excitation-contraction uncoupling: Major role in contraction-induced muscle injury. Exerc. Sport Sci. Rev. 2001, 29, 82–87. [Google Scholar] [CrossRef]
- Novelli, G.P.; Bracciotti, G.; Falsini, S. Spin-trappers and vitamin E prolong endurance to muscle fatigue in mice. Free Radic. Biol. Med. 1990, 8, 9–13. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.G.; Li, X.H. A Chinese herbal decoction, Danggui Buxue Tang, improves chronic fatigue syndrome induced by food restriction and forced swimming in rats. Phytother. Res. 2011, 25, 1825–1832. [Google Scholar] [CrossRef]
- Gui, D.; Guo, Y.; Wang, F.; Liu, W.; Chen, J.; Chen, Y.; Huang, J.; Wang, N. Astragaloside IV, a novel antioxidant, prevents glucose-induced podocyte apoptosis in vitro and in vivo. PLoS One 2012, 7, e39824. [Google Scholar]
- Sahlin, K.; Tonkonogi, M.; Söderlund, K. Energy supply and muscle fatigue in humans. Acta Physiol. Scand. 1998, 162, 261–266. [Google Scholar] [CrossRef]
- Young, A.J.; Castellani, J.W. Exertion-induced fatigue and thermoregulation in the cold. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 128, 769–776. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M. Effects of various Eleutherococcus senticosus cortex on swimming time, natural killer activity and corticosterone level in forced swimming stressed mice. J. Ethnopharmacol. 2004, 95, 447–453. [Google Scholar] [CrossRef]
- Cairns, S.P. Lactic acid and exercise performance: Culprit or friend? Sports Med. 2006, 36, 279–291. [Google Scholar] [CrossRef]
- Tashiro, S. Studies on alkaligenesis in tissues: I. Ammonia production in the nerve fiber during excitation. Am. J. Physiol. 1922, 60, 519–543. [Google Scholar]
- Carvalho-Peixoto, J.; Alves, R.C.; Cameron, L.C. Glutamine and carbohydrate supplements reduce ammonemia increase during endurance field exercise. Appl. Physiol. Nutr. Metab. 2007, 32, 1186–1190. [Google Scholar] [CrossRef]
- Chen, W.C.; Huang, W.C.; Chiu, C.C.; Chang, Y.K.; Huang, C.C. Whey Protein improves exercise performance and biochemical profiles in trained mice. Med. Sci. Sports Exerc. 2014. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ishihara, K.; Tanaka, K.; Inoue, K.; Fushiki, T. An adjustable-current swimming pool for the evaluation of endurance capacity of mice. J. Appl. Physiol. 1985, 81, 1843–1849. [Google Scholar]
- Wang, S.Y.; Huang, W.C.; Liu, C.C.; Wang, M.F.; Ho, C.S.; Huang, W.P.; Hou, C.C.; Chuang, H.L.; Huang, C.C. Pumpkin (Cucurbita. moschata) fruit extract improves physical fatigue and exercise performance in mice. Molecules 2012, 17, 11864–11876. [Google Scholar] [CrossRef]
- Wu, R.E.; Huang, W.C.; Liao, C.C.; Chang, Y.K.; Kan, N.W.; Huang, C.C. Resveratrol protects against physical fatigue and improves exercise performance in mice. Molecules 2013, 18, 4689–4702. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yeh, T.-S.; Chuang, H.-L.; Huang, W.-C.; Chen, Y.-M.; Huang, C.-C.; Hsu, M.-C. Astragalus membranaceus Improves Exercise Performance and Ameliorates Exercise-Induced Fatigue in Trained Mice. Molecules 2014, 19, 2793-2807. https://doi.org/10.3390/molecules19032793
Yeh T-S, Chuang H-L, Huang W-C, Chen Y-M, Huang C-C, Hsu M-C. Astragalus membranaceus Improves Exercise Performance and Ameliorates Exercise-Induced Fatigue in Trained Mice. Molecules. 2014; 19(3):2793-2807. https://doi.org/10.3390/molecules19032793
Chicago/Turabian StyleYeh, Tzu-Shao, Hsiao-Li Chuang, Wen-Ching Huang, Yi-Ming Chen, Chi-Chang Huang, and Mei-Chich Hsu. 2014. "Astragalus membranaceus Improves Exercise Performance and Ameliorates Exercise-Induced Fatigue in Trained Mice" Molecules 19, no. 3: 2793-2807. https://doi.org/10.3390/molecules19032793
APA StyleYeh, T.-S., Chuang, H.-L., Huang, W.-C., Chen, Y.-M., Huang, C.-C., & Hsu, M.-C. (2014). Astragalus membranaceus Improves Exercise Performance and Ameliorates Exercise-Induced Fatigue in Trained Mice. Molecules, 19(3), 2793-2807. https://doi.org/10.3390/molecules19032793