First Chemical Evaluation and Toxicity of Casinga-cheirosa to Balb-c Male Mice
Abstract
:1. Introduction
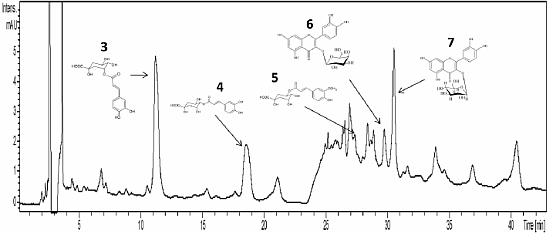
2. Results and Discussion


Discussion



| Peak No | Substance | Retention Time (min) | Molecular Weight (Da) | [M−H]− m/z |
|---|---|---|---|---|
| 3 | 3 | 11.2–11.8 | 354 | 353 |
| 4 | 4 | 18.7–19.1 | 354 | 353 |
| 5 | 5 | 27.7 | 368 | 367 |
| 6 | 6 | 30.0 | 464 | 463 |
| 7 | 7 | 30.8 | 464 | 463 |
3. Experimental
3.1. Plant Collection and Extract Preparation
3.2. Preparation of Extract to Be Administered
3.3. Animals
3.4. Acute Toxicity Signs and Delayed Death Observations
3.5. Toxicology Experimental Design
3.6. EB719 Dosing for Stage 1 and Stage 2 Toxicity Assays
3.7. Statistical Analysis
3.8. Extract Fractionation and Isolation of Compounds
Liquid-Liquid Partition
3.9. Analytical LC-DAD and Semi-Preparative LC-UV Analysis
3.10. Liquid Chromatography—Mass Spectrometry (HPLC/ESI-MSn) Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Srivastava, V.; Neqi, A.S.; Kumar, J.K.; Gupta, M.M.; Khanuja, S.P. Plant-based anticancer molecules: A chemical and biological profile of some important leads. Bioorg. Med. Chem. 2005, 13, 5892–908. [Google Scholar] [CrossRef]
- Suffredini, I.B.; Paciencia, M.L.B.; Frana, S.A.; Varella, A.D.; Younes, R.N. In vitro breast cancer cell lethality by Brazilian plant extracts. Pharmazie 2007, 62, 798–800. [Google Scholar]
- Suffredini, I.B.; Paciencia, M.L.B.; Varella, A.D.; Younes, R.N. In vitro cytotoxic activity of Brazilian plant extracts against a human lung, colon and CNS solid cancers and leukemia. Fitoterapia 2007, 78, 223–226. [Google Scholar] [CrossRef]
- Suffredini, I.B.; Varella, A.D.; Younes, R.N. Cytotoxic molecules from natural sources. Tapping the Brazilian biodiversity. Anticancer Agents Med. Chem. 2006, 6, 367–375. [Google Scholar] [CrossRef]
- Gama, J.R.V.; de Souza, A.L.; Calegário, N.; Lana, G.C.R. Fitossociologia de duas fitocenoses de floresta ombrófila aberta no município de Codó, estado do Maranhão. Rev. Árvore 2007, 31, 465–477. [Google Scholar] [CrossRef]
- Revilla, R. Plantas Úteis da Bacia Amazônica; Instituto Nacional de Pesquisas da Amazônia e Sebrae: Manaus, Brazil, 2002; Volume 1, p. 371. [Google Scholar]
- Suffredini, I.B.; Paciencia, M.L.B.; Varella, A.D.; Younes, R.N. In vitro prostate cell cancer cell growth inhibition by Brazilian plant extract. Pharmazie 2006, 61, 722–724. [Google Scholar]
- Ozi, J.M.; Suffredini, I.B.; Paciencia, M.L.B.; Frana, S.F.; Dib, L.L. In vitro cytotoxic effects of Brazilian plant extracts on squamous cell carcinoma of the oral cavity. Br. Oral Res. 2011, 25, 519–525. [Google Scholar] [CrossRef]
- Barros, S.B.M.; Davino, S.C. Avaliação da Toxicidade. In Fundamentos de Toxicologia, 3rd ed.; Oga, S., Camargo, M.M.A., Batistuzzo, J.A.O., Eds.; Atheneu: São Paulo, Brazil, 2008; pp. 59–70. [Google Scholar]
- Henry, G.E.; Adams, L.S.; Rosales, J.C.; Jacobs, H.; Heber, D.; Seeram, N.P. Kaurene diterpenes from Laetia. thamnia inhibit the growth of human cancer cell lines in vitro. Cancer Lett. 2006, 244, 190–194. [Google Scholar] [CrossRef]
- Beutler, J.A.; McCall, K.L.; Herbert, K.; Johnson, T.; Shoemaker, R.H.; Boyd, M.R. Cytotoxic clerodane diterpene esters from Laetia. corymbulosa. Phytochemistry 2000, 55, 233–236. [Google Scholar] [CrossRef]
- Jullian, V.; Bonduelle, C.; Valentin, A.; Acebey, L.; Duigou, A.G.; Prévost, M.F.; Sauvain, M. New Clerodane diterpenoids from Laetia procera (Poepp.) Eichler (Flacourtiaceae), with antiplasmoidal and antileishmanial activities. Bioorg. Med. Chem. Lett. 2005, 15, 5065–5670. [Google Scholar] [CrossRef]
- Hasani, P.; Yasa, N.; Vosough-Ghanbari, S.; Mohammadirad, A.; Dehghan, G.; Abdollahi, M. In vivo antioxidant potential of Teucrium polium, as compared to alpha-tocopherol. Acta Pharm. 2007, 57, 123–129. [Google Scholar]
- Renò, F.; Aina, V.; Gatti, S.; Cannas, M. Effect of vitamin E addition to poly(D,L)-lactic acid on surface properties and osteoblast behaviour. Biomaterials 2005, 26, 5594–5599. [Google Scholar] [CrossRef]
- Huilgol, N.G.; Nair, C.K.; Merhotra, P.; Kagiya, V.T. A phase I trial of tocopherol monoglucoside in patients undergoing hemi-body radiation. J. Cancer Res. Ther. 2005, 1, 38–40. [Google Scholar] [CrossRef]
- Alba, M.A.; Sánchez, R.R.; Pérez, N.J.; Navarrete, J.S.; Paz, R.F.; Montoya-Estrada, A.; Gómez, J.J. Comparative study of the antimutagenic properties of vitamins C and E against mutation induced by norfloxacin. BMC Pharmacol. 2008. [Google Scholar] [CrossRef]
- Bouic, P.J.D.; Etsebeth, S.; Liebenberg, R.W.; Albrecht, C.F.; Pegel, K.; van Jaarsveld, P.P. Beta-sitosterol and beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: Implications for their use as an immunomodulatory vitamin combination. Int. J. Immunopharmacol. 1996, 18, 693–700. [Google Scholar] [CrossRef]
- Khan, M.R.; Mlungwana, S.M. α-Sitosterol, a cytotoxic sterol from Markhamia zanzibarica and Kigelia africana. Fitoterapia 1999, 70, 96–97. [Google Scholar] [CrossRef]
- Awad, A.B.; Burr, A.T.; Fink, C.S. Effect of resvetratrol and β-sitosterol in combination on reactive oxygen species and prostaglandin release by PC-3 cells. Prostaglandins Leukot. Essent. Fatty Acids 2005, 72, 219–226. [Google Scholar] [CrossRef]
- Sundarraj, S.; Thangam, R.; Sreevan, V.; Kaveri, K.; Gunasekaran, P.; Achiraman, S.; Kannan, S. γ-Sitosterol from Accacia nilotica L. induces G2/M cell cycle arrest and apoptosis through c-Myc suppression in MCF-7 and A549 cells. J. Ethnopharmacol. 2012, 141, 803–809. [Google Scholar] [CrossRef]
- Romagnolo, D.F.; Selmin, O.I. Flavonoids and cancer prevention: A review of the evidence. J. Nutr. Gerontol. Geriatr. 2012, 31, 206–238. [Google Scholar] [CrossRef]
- Stich, H.F. The beneficial and hazardous effects of simple phenolic compounds. Mutat. Res. 1991, 259, 307–324. [Google Scholar] [CrossRef]
- Brito, A.S. Manual de Ensaios de Toxicologia; Editora da Unicamp: Campinas, Brazil, 1994; p. 122. [Google Scholar]
- Gusmão, D.F.; Estork, D.M.; Paciencia, M.L.B.; Diaz, I.E.C.; Frana, S.A.; Rodrigues, P.A.; Suffredini, I.B.; Varella, A.D.; Younes, R.N.; Reis, L.F.L.; et al. Preliminary evaluation of the acute toxicity related to Abarema auriculata to mice and investigation of cytotoxicity of isolated flavonones. Pharmacologyonline 2013, 1, 113–127. [Google Scholar]
- Masuda, Y.; Suzuki, M.; Akagawa, Y.; Takemura, T. Developmental and Pharmacological Features of Mouse emotional piloerection. Exp. Anim. 1999, 48, 209–211. [Google Scholar]
- Miller, L.G. Herbal medicines: Selected clinical considerations focusing on known or potential drug-herb interactions. Arch. Intern. Med. 1998, 58, 2200–2211. [Google Scholar] [CrossRef]
- Takahashi, O.; Ichikawa, H.; Sasaki, M. Hemorrhagic toxicity of d-alpha-tocopherol in the rat. Toxicology 1990, 63, 157–165. [Google Scholar] [CrossRef]
- Strauch, M.A.; Tomaz, M.A.; Monteiro-Machado, M.; Ricardo, H.D.; Cons, B.L.; Fernandes, F.F.; El-Kik, C.Z.; Azevedo, M.S.; Melo, P.A. Antiophidic activity of the extract of the Amazon plant Humirianthera ampla and consitituents. J. Ethnopharmacol. 2013, 45, 50–58. [Google Scholar]
- Nugroho, A.; Lim, S.C.; Byeon, J.S.; Choi, J.S.; Park, H.J. Simultaneous quantification and validation of caffeoylquyinic acids and flavonoids in Hemistepta lyrata and peroxynitrite-svavenging activity. J. Pharm. Biomed. Anal. 2013, 76, 139–144. [Google Scholar] [CrossRef]
- F Vale, L.H.; Mendes, M.M.; Fernandes, R.S.; Costa, T.R.; Hage-Melim, L.I.; A Sousa, M.; Hamaguchi, A.; Homsi-Brandeburgo, M.I.; Franca, S.C.; Silva, C.H.; et al. Protective effect of Schizolobium. parahyba flavonoids against snake venoms and isolated toxins. Curr. Top. Med. Chem. 2011, 11, 2566–2577. [Google Scholar] [CrossRef]
- Matsuo, M.; Urano, S. 13C-NMR Spectra of Tocopherols and 2,2-dimethylchromanols. Tetrahedron 1976, 32, 229–231. [Google Scholar] [CrossRef]
- Holland, H.L.; Diakow, P.R.P.; Taylor, G.J. 13C Nuclear magnetic resonance spectra of some C-19-hydroxy, C-5,6-epoxy, C-24-ethyl and C-19-norsteroids. Can. J. Chem. 1978, 56, 3121–3127. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Estork, D.M.; Gusmão, D.F.; Paciencia, M.L.B.; Díaz, I.E.C.; Varella, A.D.; Younes, R.N.; Reis, L.F.L.; Montero, E.F.S.; Bernardi, M.M.; Suffredini, I.B. First Chemical Evaluation and Toxicity of Casinga-cheirosa to Balb-c Male Mice. Molecules 2014, 19, 3973-3987. https://doi.org/10.3390/molecules19043973
Estork DM, Gusmão DF, Paciencia MLB, Díaz IEC, Varella AD, Younes RN, Reis LFL, Montero EFS, Bernardi MM, Suffredini IB. First Chemical Evaluation and Toxicity of Casinga-cheirosa to Balb-c Male Mice. Molecules. 2014; 19(4):3973-3987. https://doi.org/10.3390/molecules19043973
Chicago/Turabian StyleEstork, Dirce M., Daniela F. Gusmão, Mateus L. B. Paciencia, Ingrit E. C. Díaz, Antonio D. Varella, Riad N. Younes, Luiz F. L. Reis, Edna F. S. Montero, Maria M. Bernardi, and Ivana B. Suffredini. 2014. "First Chemical Evaluation and Toxicity of Casinga-cheirosa to Balb-c Male Mice" Molecules 19, no. 4: 3973-3987. https://doi.org/10.3390/molecules19043973