Optimization of Fermentation Conditions for the Production of the M23 Protease Pseudoalterin by Deep-Sea Pseudoalteromonas sp. CF6-2 with Artery Powder as an Inducer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Different Inducers on Pseudoalterin Production
| Inducers | Elastolytic activity after different fermentation time (U/mL) | |||||
|---|---|---|---|---|---|---|
| 24 h | 36 h | 48 h | 60 h | 72 h | 84 h | |
| Elastin (0.3%) | 16.5 ± 0.7 | 32.4 ± 4.0 | 35.3 ± 3.5 | 42.9 ± 3.2 | 46.9 ± 3.9 | 41.4 ± 3.6 |
| Artery (wet, 2.5%) | 12.6 ± 0.8 | 27.5 ± 1.0 | 42.1 ± 2.0 | 45.8 ± 1.6 | 54.6 ± 2.4 | 42.7 ± 1.7 |
| Bovine lung (wet, 2.5%) | 6.2 ± 0.8 | 9.2 ± 1.7 | 7.1 ± 1.2 | 7.2 ± 1.3 | 14.5 ± 1.4 | 11.4 ± 0.6 |
| Ligament (wet, 2.5%) | 10.3 ± 0.4 | 18.0 ± 0.9 | 11.9 ± 0.6 | 10.0 ± 0.7 | 18.1 ± 0.7 | 16.2 ± 1.2 |
| Chicken intestine (wet, 2.5%) | 6.7 ± 1.2 | 9.7 ± 1.0 | 8.3 ± 0.4 | 7.2 ± 0.4 | 14.9 ± 1.0 | 11.1 ± 1.0 |
| Beef extract (1%) | 4.8 ± 0.5 | 11.6 ± 0.4 | 11.2 ± 0.0 | 14.2 ± 0.8 | 13.1 ± 0.9 | 12.9 ± 1.3 |
| Gelatin (1%) | ND b | 4.3 ± 0.7 | 1.1 ± 0.0 | 0.9 ± 0.2 | ND b | ND b |
| Yeast extract peptone dextrose (1%) | ND b | 3.6 ± 0.2 | 2.2 ± 0.1 | 3.8 ± 0.2 | 2.4 ± 0.2 | 2.2 ± 0.2 |
| BSA (1%) | 1.1 ± 0.0 | 1.0 ± 0.0 | 1.3 ± 0.1 | 1.0 ± 0.0 | ND b | ND b |
| Peptone (1%) | ND b | 1.6 ± 0.1 | 1.3 ± 0.1 | 3.1 ± 0.2 | 1.1 ± 0.1 | 2.0 ± 0.0 |
| Soybean meal (1%) | ND b | ND b | 0.9 ± 0.0 | 1.8 ± 0.4 | 1.4 ± 0.4 | 2.2 ± 0.2 |
| Casein (1%) | ND b | 3.6 ± 0.3 | 3.1 ± 0.5 | 5.0 ± 0.1 | 1.4 ± 0.0 | 2.9 ± 0.2 |
2.2. Single Factor Experiments

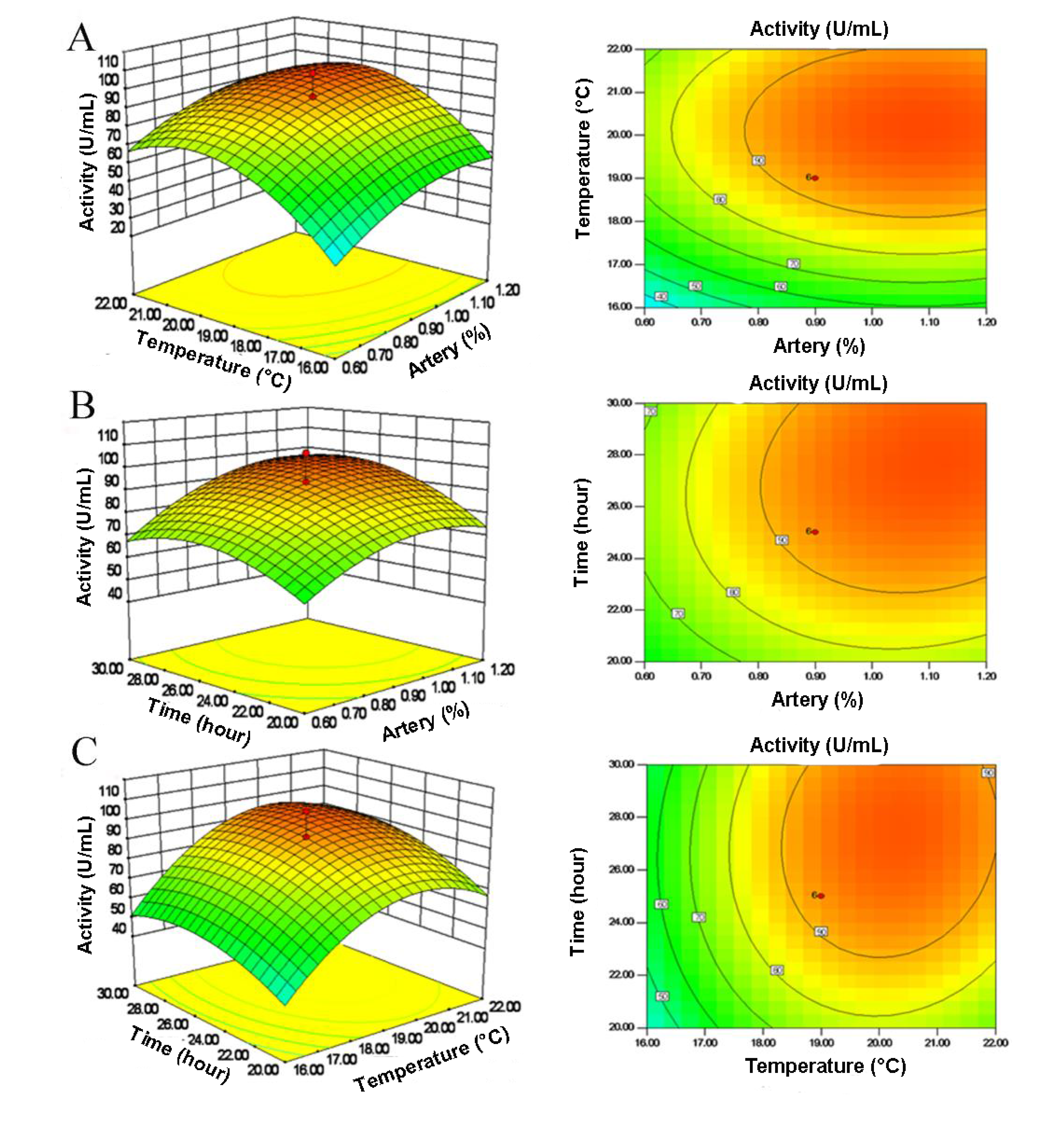
2.3. Central Composite Design (CCD) and Response Surface Analysis
| Run | X1 (artery) | X2 (temperature) | X3 (time) | Activity (U/mL) | |||
|---|---|---|---|---|---|---|---|
| Coded level | Real level (%) | Coded level | Real level (°C) | Coded level | Real Level (h) | ||
| 1 | 0 | 0.9 | +1.68 | 24.0 | 0 | 25.0 | 54.38 |
| 2 | 0 | 0.9 | 0 | 19.0 | +1.68 | 33.4 | 75.99 |
| 3 | +1 | 1.2 | +1 | 22.0 | −1 | 20.0 | 66.51 |
| 4 | +1 | 1.2 | +1 | 22.0 | +1 | 30.0 | 99.23 |
| 5 | −1 | 0.6 | +1 | 22.0 | +1 | 30.0 | 63.37 |
| 6 | 0 | 0.9 | 0 | 19.0 | 0 | 25.0 | 89.15 |
| 7 | 0 | 0.9 | 0 | 19.0 | −1.68 | 16.6 | 52.76 |
| 8 | −1 | 0.6 | −1 | 16.0 | +1 | 30.0 | 26.42 |
| 9 | 0 | 0.9 | −1.68 | 14.0 | 0 | 25.0 | 7.79 |
| 10 | 0 | 0.9 | 0 | 19.0 | 0 | 25.0 | 91.40 |
| 11 | 0 | 0.9 | 0 | 19.0 | 0 | 25.0 | 89.83 |
| 12 | +1 | 1.2 | −1 | 16.0 | +1 | 30.0 | 56.65 |
| 13 | +1.68 | 1.4 | 0 | 19.0 | 0 | 25.0 | 78.99 |
| 14 | −1 | 0.6 | +1 | 22.0 | −1 | 20.0 | 50.66 |
| 15 | +1 | 1.2 | −1 | 16.0 | −1 | 20.0 | 41.81 |
| 16 | 0 | 0.9 | 0 | 19.0 | 0 | 25.0 | 93.82 |
| 17 | −1 | 0.6 | −1 | 16.0 | −1 | 20.0 | 16.37 |
| 18 | −1.68 | 0.4 | 0 | 19.0 | 0 | 25.0 | 50.78 |
| 19 | 0 | 0.9 | 0 | 19.0 | 0 | 25.0 | 90.16 |
| 20 | 0 | 0.9 | 0 | 19.0 | 0 | 25.0 | 106.42 |
| Source | df | Elastolytic activity of fermented broth a | |||
|---|---|---|---|---|---|
| Sum of Squares | Mean Square | F Value | p-value Prob > F | ||
| Model | 9 | 14610.93 | 1623.44 | 41.99 | <0.0001 b |
| X1 | 1 | 1755.06 | 1755.06 | 45.39 | <0.0001 b |
| X2 | 1 | 3455.08 | 3455.08 | 89.36 | <0.0001 b |
| X3 | 1 | 876.13 | 876.13 | 22.66 | 0.0008 b |
| X1X2 | 1 | 1.97 | 1.97 | 0.051 | 0.8258 |
| X1X3 | 1 | 76.86 | 76.86 | 1.99 | 0.1889 |
| X2X3 | 1 | 52.68 | 52.68 | 1.36 | 0.2702 |
| X12 | 1 | 1371.88 | 1371.88 | 35.48 | 0.0001 b |
| X22 | 1 | 6815.04 | 6815.04 | 176.25 | <0.0001 b |
| X32 | 1 | 1423.06 | 1423.06 | 36.80 | 0.0001 b |
| Residual | 10 | 386.67 | 38.67 | ||
| Lack of Fit | 5 | 171.62 | 34.32 | 0.80 | 0.5947 |
| Pure Error | 5 | 215.05 | 43.01 | ||
| Cor Total | 19 | 14997.60 | |||
2.4. Verification of Optimal Conditions

3. Experimental Section
3.1. Strain and Media
3.2. Inoculum Preparation and Flask Fermentation
3.3. Determination of Elastase Activity
3.4. Effect of Different Inducers on Pseudoalterin Production
3.5. Optimization by Single Factor Experiments
3.6. Optimization by Response Surface Methodology (RSM)
3.7. Statistical Analysis

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rawlings, N.D.; Barrett, A.J.; Bateman, A. MEROPS: The peptidase database. Nucleic Acids Res. 2010, 38, D227–D233. [Google Scholar] [CrossRef]
- Ahmed, K.; Chohnan, S.; Ohashi, H.; Hirata, T.; Masaki, T.; Sakiyama, F. Purification, bacteriolytic activity, and specificity of beta-lytic protease from Lysobacter sp. IB-9374. J. Biosci. Bioeng. 2003, 95, 27–34. [Google Scholar] [CrossRef]
- Kessler, E. Beta-lytic metalloendopeptidase. In Handbook of Proteolytic Enzymes, 2nd ed.; Elsevier: London, UK, 2004; pp. 998–1000. [Google Scholar]
- Kessler, E.; Ohman, D. Pseudolysin. In Handbook of Proteolytic Enzymes, 2nd ed.; Elsevier: London, UK, 2004; pp. 1001–1003. [Google Scholar]
- Kessler, E.; Safrin, M.; Abrams, W.R.; Rosenbloom, J.; Ohman, D.E. Inhibitors and specificity of Pseudomonas aeruginosa LasA. J. Biol. Chem. 1997, 272, 9884–9889. [Google Scholar]
- Park, P.W.; Mecham, R.P. Lysostaphin. In Handbook of Proteolytic Enzymes, 2nd ed.; Elsevier: London, UK, 2004; pp. 1004–1005. [Google Scholar]
- Vessillier, S.; Delolme, F.; Bernillon, J.; Saulnier, J.; Wallach, J. Hydrolysis of glycine-containing elastin pentapeptides by LasA, a metalloelastase from Pseudomonas aeruginosa. Eur. J. Biochem. 2001, 268, 1049–1057. [Google Scholar] [CrossRef]
- Rosenbloom, J.; Abrams, W.R.; Mecham, R. Extracellular matrix 4. The elastic fiber. FASEB J. 1993, 7, 1208–1218. [Google Scholar]
- Kessler, E.; Safrin, M.; Olson, J.C.; Ohman, D.E. Secreted LasA of Pseudomonas aeruginosa is a staphylolytic protease. J. Biol. Chem. 1993, 268, 7503–7508. [Google Scholar]
- Barequet, I.S.; Ben Simon, G.J.; Safrin, M.; Ohman, D.E.; Kessler, E. Pseudomonas aeruginosa LasA protease in treatment of experimental staphylococcal keratitis. Antimicrob. Agents Chemother. 2004, 48, 1681–1687. [Google Scholar] [CrossRef]
- Barequet, I.S.; Habot-Wilner, Z.; Mann, O.; Safrin, M.; Ohman, D.E.; Kessler, E.; Rosner, M. Evaluation of Pseudomonas aeruginosa staphylolysin (LasA protease) in the treatment of methicillin-resistant Staphylococcus aureus endophthalmitis in a rat model. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 913–917. [Google Scholar] [CrossRef]
- Donovan, D.M. Bacteriophage and peptidoglycan degrading enzymes with antimicrobial applications. Recent Pat. Biotechnol. 2007, 1, 113–122. [Google Scholar] [CrossRef]
- Zhou, M.Y.; Chen, X.L.; Zhao, H.L.; Dang, H.Y.; Luan, X.W.; Zhang, X.Y.; He, H.L.; Zhou, B.C.; Zhang, Y.Z. Diversity of both the cultivable protease-producing bacteria and their extracellular proteases in the sediments of the South China sea. Microb. Ecol. 2009, 58, 582–590. [Google Scholar] [CrossRef]
- Zhao, H.L.; Chen, X.L.; Xie, B.B.; Zhou, M.Y.; Gao, X.; Zhang, X.Y.; Zhou, B.C.; Weiss, A.S.; Zhang, Y.Z. Elastolytic Mechanism of a Novel M23 Metalloprotease Pseudoalterin from Deep-sea Pseudoalteromonas sp. CF6-2: Cleaving not only glycyl bonds in the hydrophobic regions but also peptide bonds in the hydrophilic regions involved in cross-linking. J. Biol. Chem. 2012, 287, 39710–39720. [Google Scholar] [CrossRef]
- Chen, X.L.; Xie, B.B.; Bian, F.; Zhao, G.Y.; Zhao, H.L.; He, H.L.; Zhou, B.C.; Zhang, Y.Z. Ecological function of myroilysin, a novel bacterial M12 metalloprotease with elastinolytic activity and a synergistic role in collagen hydrolysis, in biodegradation of deep-sea high molecular weight organic nitrogen. Appl. Environ. Microbiol. 2009, 75, 1838–1844. [Google Scholar] [CrossRef]
- Kiviharju, K.; Leisola, M.; Eerikäinen, T. Optimization of Streptomyces peucetius var. caesius N47 cultivation and ε-rhodomycinone production using experimental designs and response surface methods. J. Ind. Microbiol. Biotechnol. 2004, 31, 475–481. [Google Scholar] [CrossRef]
- Chu, W.H. Optimization of extracellular alkaline protease production from species of Bacillus. J. Ind. Microbiol. Biotechnol. 2007, 34, 241–245. [Google Scholar] [CrossRef]
- Liu, C.; Sun, Z.T.; Du, J.H.; Wang, J. Response surface optimization of fermentation conditions for producing xylanase by Aspergillus niger SL-05. J. Ind. Microbiol. Biotechnol. 2008, 35, 703–711. [Google Scholar] [CrossRef]
- He, Y.Q.; Tan, T.W. Use of response surface methodology to optimize culture medium for production of lipase with Candida sp. 99–125. J. Mol. Catal. B Enzym. 2006, 43, 9–14. [Google Scholar] [CrossRef]
- Liu, S.B.; Qiao, L.P.; He, H.L.; Zhang, Q.; Chen, X.L.; Zhou, W.Z.; Zhou, B.C.; Zhang, Y.Z. Optimization of fermentation conditions and rheological properties of exopolysaccharide produced by deep-sea bacterium Zunongwangia profunda SM-A87. PLoS One 2011, 6, e26825. [Google Scholar]
- Muralidhar, R.V.; Chirumamila, R.R.; Marchant, R.; Nigam, P. A response surface approach for the comparison of lipase production by Candida cylindracea using two different carbon sources. Biochem. Eng. J. 2001, 9, 17–23. [Google Scholar] [CrossRef]
- Xu, H.; Sun, L.P.; Shi, Y.Z.; Wu, Y.H.; Zhang, B.; Zhao, D.Q. Optimization of cultivation conditions for extracellular polysaccharide and mycelium biomass by Morchella esculenta As51620. Biochem. Eng. J. 2008, 39, 66–73. [Google Scholar] [CrossRef]
- Song, X.Y.; Xie, S.T.; Chen, X.L.; Sun, C.Y.; Shi, M.; Zhang, Y.Z. Solid-state fermentation for Trichokonins production from Trichoderma koningii SMF2 and preparative purification of Trichokonin VI by a simple protocol. J. Biotechnol. 2007, 131, 209–215. [Google Scholar] [CrossRef]
- Triveni, R.; Shamala, T.R.; Rastogi, N.K. Optimised production and ultilization of exopolysaccharide from Agrobacterium radiobacter. Process Biochem. 2001, 36, 787–795. [Google Scholar] [CrossRef]
- Design-Expert software, version 8.0.4, A software for experiment design; Stat-Ease Inc: Minneapolis, MN, USA, 2010.
- Sample Availability: Samples of the compound pseudoalterin are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhao, H.-L.; Yang, J.; Chen, X.-L.; Su, H.-N.; Zhang, X.-Y.; Huang, F.; Zhou, B.-C.; Xie, B.-B. Optimization of Fermentation Conditions for the Production of the M23 Protease Pseudoalterin by Deep-Sea Pseudoalteromonas sp. CF6-2 with Artery Powder as an Inducer. Molecules 2014, 19, 4779-4790. https://doi.org/10.3390/molecules19044779
Zhao H-L, Yang J, Chen X-L, Su H-N, Zhang X-Y, Huang F, Zhou B-C, Xie B-B. Optimization of Fermentation Conditions for the Production of the M23 Protease Pseudoalterin by Deep-Sea Pseudoalteromonas sp. CF6-2 with Artery Powder as an Inducer. Molecules. 2014; 19(4):4779-4790. https://doi.org/10.3390/molecules19044779
Chicago/Turabian StyleZhao, Hui-Lin, Jie Yang, Xiu-Lan Chen, Hai-Nan Su, Xi-Ying Zhang, Feng Huang, Bai-Cheng Zhou, and Bin-Bin Xie. 2014. "Optimization of Fermentation Conditions for the Production of the M23 Protease Pseudoalterin by Deep-Sea Pseudoalteromonas sp. CF6-2 with Artery Powder as an Inducer" Molecules 19, no. 4: 4779-4790. https://doi.org/10.3390/molecules19044779





