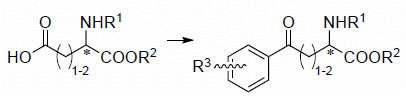
Utilization of Acidic α-Amino Acids as Acyl Donors: An Effective Stereo-Controllable Synthesis of Aryl-Keto α-Amino Acids and Their Derivatives
Abstract
:1. Introduction

2. General Methods to Prepare Aryl-keto α-Amino Acid Derivatives
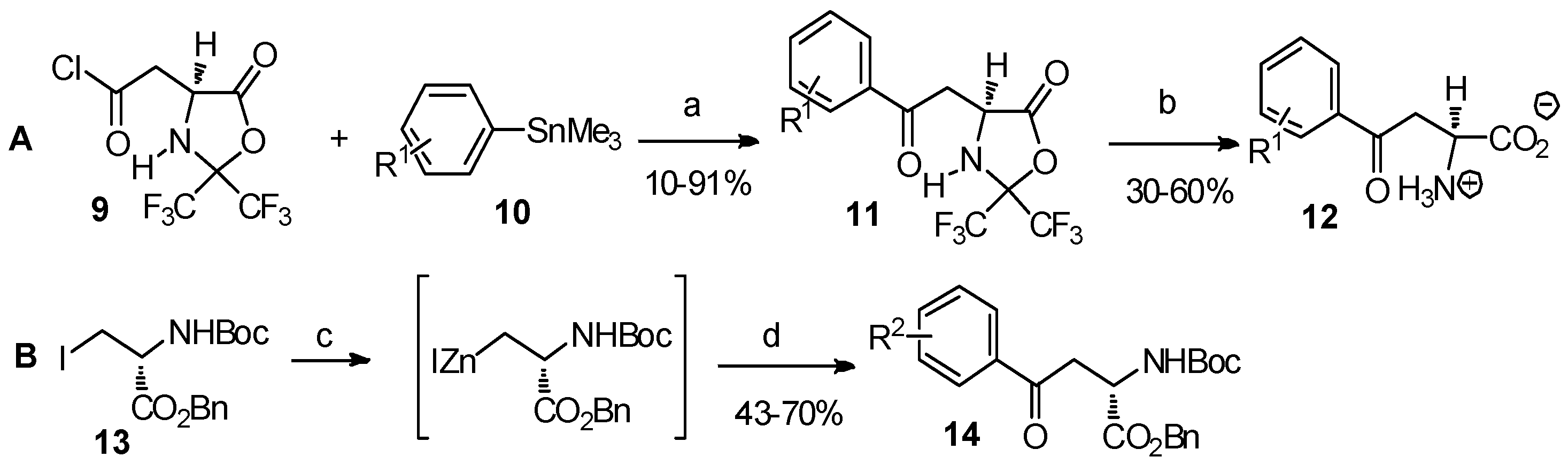
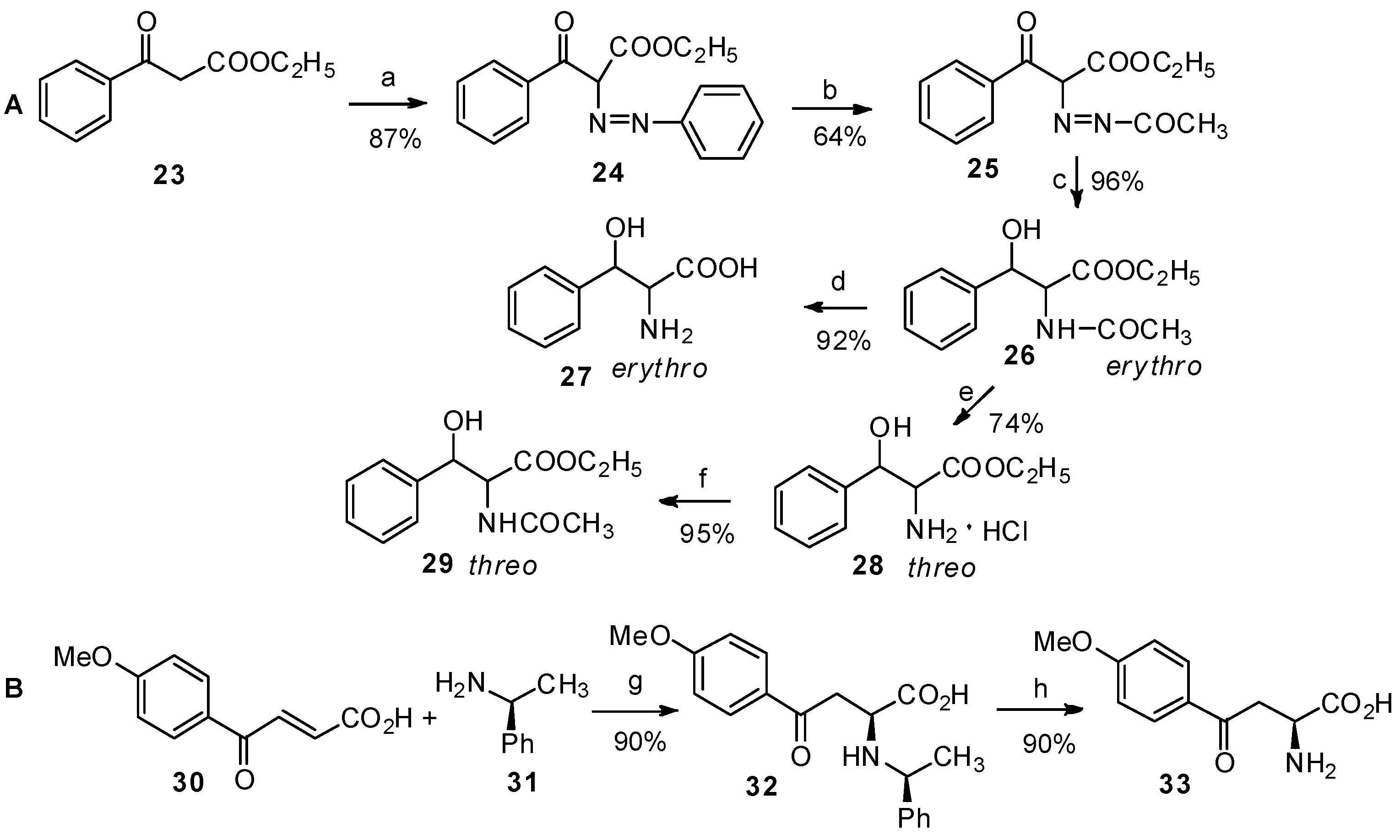
2.1. α-Amino Acid Derivatives as the Reactants


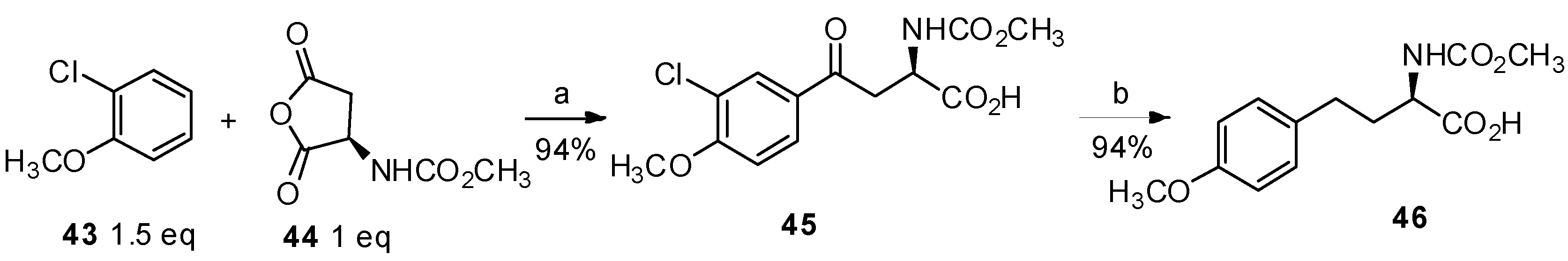
2.2. Non-Amino Acid Derivatives as the Reactants
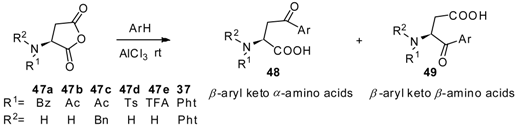
3. Preparation of Aryl Keto α-amino Acids by Friedel-Crafts Acylation
3.1. AlCl3 Catalyzed Friedel-Crafts Acylation



| Substrate | arene | Time (h) | α/β-amino acids ratio | Yield (%) | Substrate | arene | Time (h) | α/β-amino acids ratio | Yield (%) |
|---|---|---|---|---|---|---|---|---|---|
| 47a | benzene | 5 | 55/45 | 51 | 47c | toluene | 5 | 7/93 | 47 |
| 47a | toluene | 5 | 39/61 | 33 | 47c | o-xylene | 15 | 39/61 | 52 |
| 47a | o-xylene | 15 | 30/70 | 65 | 47d | benzene | 5 | 84/16 | 76 |
| 47a | mesitylene | 15 | 100/0 | 51 | 47d | toluene | 5 | 100/0 | 71 |
| 47a | anisole | 72 | 50/50 | 31 | 47d | o-xylene | 15 | 74/26 | 83 |
| 47a | veratrole | 96 | 100/0 | 18 | 47e | benzene | 5 | 95/5 | 78 |
| 47a | naphthalene | 15 | 95/5 | 44 | 47e | toluene | 5 | 80/20 | 62 |
| 47b | benzene | 5 | 95/5 | 76 | 47e | o-xylene | 15 | 56/44 | 70 |
| 47b | toluene | 5 | 64/36 | 52 | 37 | benzene | 5 | 100/0 | 64 |
| 47b | o-xylene | 15 | 58/42 | 67 | 37 | toluene | 5 | 88/12 | 71 |
| 47c | benzene | 5 | 30/70 | 55 | 37 | o-xylene | 15 | 48/52 | 83 |

3.2. HF Catalyzed Friedel-Crafts Acylation
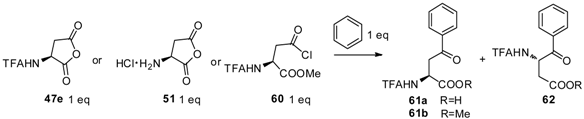
3.3. TfOH Catalyzed Friedel-Crafts Acylation

| Entry | Donor | Catalyst (eq) | Solvent | Reaction Time (h) | Product Yiled (%) |
|---|---|---|---|---|---|
| 1 | 47e | AlCl3 (8) | CH2Cl2 | 1 or 12 | 0 |
| 2 | 47e | TiCl4 (90) | neat | 1 or 12 | 1 |
| 3 | 47e | H2SO4 (90) | neat | 1 or 12 | 2 |
| 4 | 47e | TfOH (40) | neat | 1 | 61a (52), 62 (3) |
| 5 | 47e | Tf2OH (25) | neat | 1 or 12 | 0 |
| 6 | 51 | TfOH (40) | neat | 1 | 0 |
| 7 | 60 | AlCl3 (8) | CH2Cl2 | 10 | 61b (50) |
| 8 | 60 | TiCl4 (90) | neat | 1 or 12 | 0 |
| 9 | 60 | H2SO4 (90) | neat | 1 or 12 | 0 |
| 10 | 60 | TfOH (40) | neat | 1 | 61b (98) |
| 11 | 60 | Tf2OH (25) | neat | 1 or 12 | 0 |







4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hartung, W.H.; Dittrich, T.T.; Chang, Y.T. β-Arylserine ethyl esters. J. Am. Chem. Soc. 1953, 75, 238–239. [Google Scholar] [CrossRef]
- Hernández, D.; Vilar, G.; Riego, E.; Cañedo, L.M.; Cuevas, C.; Albericio, F.; Álvarez, M. Synthesis of IB-01211, a cyclic peptide containing 2,4-concatenated thia- and oxazoles, via Hantzsch macrocyclization. Org. Lett. 2007, 9, 809–811. [Google Scholar]
- Ojika, M.; Inukai, Y.; Kito, Y.; Hirata, M.; Iizuka, T.; Fudou, R. Miuraenamides: Antimicrobial cyclic depsipeptides isolated from a rare and slightly halophilic myxobacterium. Chem. Asian J. 2008, 3, 126–133. [Google Scholar] [CrossRef]
- Vanderhaeghe, H.; Parmentier, G. The structure of factor S of staphylomycin. J. Am. Chem. Soc. 1960, 82, 4414–4422. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yamaki, H.; Shinoda, T.; Tago, Y.; Suzuki, H.; Nishimura, T.; Yamaguchi, H. The mode of antifungal action of (S)2-amino-4-oxo-5-hydroxypentanoic acid, RI-331. J. Antibiot. 1990, 43, 411–416. [Google Scholar] [CrossRef]
- Kumar, S.; Gawandi, V.B.; Capito, N.; Phillips, R.S. Substituent effects on the reaction of β-benzoylalanines with Pseudomonas fluorescens kynureninase. Biochemistry 2010, 49, 7913–7919. [Google Scholar] [CrossRef]
- Weiβ, T.D.; Helmchen, G.; Kazmaier, U. Synthesis of amino acid derivatives via enantio- and diastereoselective Pd-catalyzed allylic substitutions with a non-stabilized enolate as nucleophile. Chem. Commun. 2002, 2002, 1270–1271. [Google Scholar]
- Arend, M. Asymmetric catalytic aminoalkylations: New powerful methods for the enantioselective synthesis of amino acid derivatives, Mannich bases, and homoallylic amines. Angew. Chem. Int. Ed. 1999, 38, 2873–2874. [Google Scholar] [CrossRef]
- Ferraris, D.; Young, B.; Dudding, T.; Lectka, T. Catalytic, enantioselective alkylation of α-imino esters using late transition metal phosphine complexes as catalysts. J. Am. Chem. Soc. 1998, 120, 4548–4549. [Google Scholar] [CrossRef]
- Yamada, M.; Nagashima, N.; Hasegawa, J.; Takahashi, S. A highly efficient asymmetric synthesis of methoxyhomophenylalanine using Michael addition of phenethylamine. Tetrahedron Lett. 1998, 39, 9019–9022. [Google Scholar] [CrossRef]
- Gore, P.H. The Friedel-Crafts acylation reaction and its application to polycyclic aromatic hydrocarbons. Chem. Rev. 1955, 55, 229–281. [Google Scholar] [CrossRef]
- Lin, W.; He, Z.; Zhang, H.; Zhang, X.; Mi, A.; Jiang, Y. Amino acid anhydride hydrochlorides as acylating agents in Friedel-Crafts reaction: A practical synthesis of L-homophenylalanine. Synthesis 2001, 1007–1009. [Google Scholar]
- Melillo, D.G.; Larsen, R.D.; Mathre, D.J.; Shukis, W.F.; Wood, A.W.; Colleluori, J.R. Practical enantioselective synthesis of a homotyrosine derivative and (R,R)-4-propyl-9-hydroxynaphthoxazine, a potent dopamine agonist. J. Org. Chem. 1987, 52, 5143–5150. [Google Scholar] [CrossRef]
- Murai, Y.; Hatanaka, Y.; Kanaoka, Y.; Hashimoto, M. Effective synthesis of optically active 3-phenyl-3-(3-trifluoromethyl)diazirinyl bishomophenylalanine derivatives. Heterocycles 2009, 79, 359–364. [Google Scholar] [CrossRef]
- Huckin, S.N.; Weiler, L. Claisen condensation of the dianion of β-keto esters. Tetrahedron Lett. 1972, 13, 2405–2408. [Google Scholar] [CrossRef]
- Singh, J.; Gordon, T.D.; Earley, W.G.; Morgan, B.A. An efficient synthesis and acylation of α-amino-β-keto-esters: Versatile intermediates in the synthesis of peptide mimetics. Tetrahedron Lett. 1993, 34, 211–214. [Google Scholar] [CrossRef]
- Suzuki, M.; Iwasaki, T.; Miyoshi, M.; Okumura, K.; Matsumoto, K. New convenient synthesis of α-C-acylamino acids and α-amino ketones. J. Org. Chem. 1973, 38, 3571–3575. [Google Scholar] [CrossRef]
- Schultz, K.; Stief, L.; Kazmaier, U. A straightforward approach towards α-amino-β-keto esters via acylation of chelated amino acid ester enolates. Synthesis 2012, 600–604. [Google Scholar]
- Golubev, A.S.; Sewald, N.; Burger, K. Synthesis of γ-oxo α-amino acids from L-aspartic acid. Tetrahedron 1996, 52, 14757–14776. [Google Scholar] [CrossRef]
- Jackson, R.F.W.; Wishart, N.; Wood, A.; James, K.; Wythes, M.J. Preparation of enantiomerically pure protected 4-oxo α-amino acids and 3-aryl α-amino acids from serine. J. Org. Chem. 1992, 57, 3397–3404. [Google Scholar] [CrossRef]
- Haudegond, J.P.; Chauvin, Y.; Commereuc, D. Synthesis of α-amino acids by alkylation of diethyl acetamidomalonate in the presence of palladium complexes. J. Org. Chem. 1979, 44, 3063–3065. [Google Scholar] [CrossRef]
- Leanna, M.R.; Morton, H.E. N-(Boc)-L-(2-Bromoallyl)-glycine: A versatile intermediate for the synthesis of optically active unnatural amino acids. Tetrahedron Lett. 1993, 34, 4485–4488. [Google Scholar]
- Kotha, S.; Singh, K. N-Alkylation of diethyl acetamidomalonate: Synthesis of constrained amino acid derivatives by ring-closing metathesis. Tetrahedron Lett. 2004, 45, 9607–9610. [Google Scholar] [CrossRef]
- Watanabe, L.A.; Jose, B.; Kato, T.; Nishino, N.; Yoshida, M. Synthesis of L-α-amino-ω-bromoalkanoic acid for side chain modification. Tetrahedron Lett. 2004, 45, 491–494. [Google Scholar] [CrossRef]
- Gawandi, V.B.; Liskey, D.; Lima, S.; Phillips, R.S. Reaction of Pseudomonas fluorescens kynureninase with β-benzoyl-L-alanine: Detection of a new reaction intermediate and a change in rate-determining step. Biochemistry 2004, 43, 3230–3237. [Google Scholar] [CrossRef]
- Varasi, M.; Torre, A.D.; Heidempergher, F.; Pevarello, P.; Speciale, C.; Guidetti, P.; Wells, D.R.; Schwarcz, R. Derivatives of kynurenine as inhibitors of rat brain kynurenine aminotransferase. Eur. J. Med. Chem. 1996, 31, 11–21. [Google Scholar] [CrossRef]
- Schmidt, U.; Griesser, H.; Lieberknecht, A.; Schmidt, J.; Gräther, T. Synthesis of α-acylamino-β-oxo acid esters. Synthesis 1993, 1993, 765–766. [Google Scholar] [CrossRef]
- Pines, S.H.; Karady, S.; Sletzinger, M. 3-Aryl-2-methylserines. I. A new synthesis. J. Org. Chem. 1968, 33, 1758–1761. [Google Scholar] [CrossRef]
- Berrée, F.; Chang, K.; Cobas, A.; Rapoport, H. Synthesis of anisolylated aspartyl and glutamyl tripeptides. J. Org. Chem. 1996, 61, 715–721. [Google Scholar]
- Thomas, H.A.; Ling, N.; Wei, E.T.; Berree, F.; Cobas, A.; Rapoport, H. Novel anti-inflammatory undecapeptides that contain anisolylated glutamic acid derivatives. J. Pharmacol. Exp. Ther. 1993, 267, 1321–1326. [Google Scholar]
- Münster, P.; Steglich, W. Synthesis of α-amino acids by reaction of t-butyl N-(t-butoxycarbonyl)iminoacetate with C-nucleophiles. Synthesis 1987, 1987, 223–225. [Google Scholar] [CrossRef]
- Kleijn, L.H.J.; Müskens, F.M.; Oppedijk, S.F.; Bruin, G.D.; Martin, N.I. A concise preparation of the non-proteinogenic amino acid L-kynurenine. Tetrahedron Lett. 2012, 53, 6430–6432. [Google Scholar] [CrossRef]
- Bretschneider, T.; Miltz, W.; Münster, P.; Steglich, W. New syntheses of α-amino acids based on N-acylimino acetates. Tetrahedron 1988, 44, 5403–5414. [Google Scholar] [CrossRef]
- Harding, K.E.; Moreno, L.N.; Nace, V.M. Hydroxyalkylation and acylation reactions of methyl hippurate. J. Org. Chem. 1981, 46, 2809–2812. [Google Scholar] [CrossRef]
- O’Donnell, M.J.; Bennett, W.D. The synthesis of amino acids by reaction of an electrophilic glycine cation equivalent with neutral carbon nucleophiles. Tetrahedron 1988, 44, 5389–5401. [Google Scholar] [CrossRef]
- Bolhofer, W.A. β-Phenylserine. J. Am. Chem. Soc. 1952, 74, 5459–5461. [Google Scholar] [CrossRef]
- Barfoot, C.W.; Harvey, J.E.; Kenworthy, M.N.; Kilburn, J.P.; Ahmed, M.; Taylor, R.J.K. Highly functionalised organolithium and organoboron reagents for the preparation of enantiomerically pure α-amino acids. Tetrahedron 2005, 61, 3403–3417. [Google Scholar] [CrossRef]
- Hara, O.; Ito, M.; Hamada, Y. Novel N → C acyl migration reaction of acyclic imides: A facile method for α-aminoketones and β-aminoalcohols. Tetrahedron Lett. 1998, 39, 5537–5540. [Google Scholar] [CrossRef]
- Olsen, R.K.; Feng, X. Synthesis of oxazolidine derivatives of β-[3-(aryloxy)aryl]-α-amino acids by application of the Diels-Alder reaction. Tetrahedron Lett. 1991, 32, 5721–5724. [Google Scholar] [CrossRef]
- Chacko, S.; Ramapanicker, R. Synthesis of γ-oxo γ-aryl and γ-aryl α-amino acids from aromatic aldehydes and serine. Eur. J. Org. Chem. 2012, 2012, 7120–7128. [Google Scholar] [CrossRef]
- Price, C.C. Alkylation and Related Reactions. In Friedel-Crafts and Related Reactions; Olah, G.A., Ed.; Interscience (Wiley): New York, NY, USA, 1964; volume 145, pp. 1174–1175. [Google Scholar]
- Kangani, C.O.; Day, B.W. Mild, efficient Friedel−Crafts acylations from carboxylic acids using cyanuric chloride and AlCl3. Org. Lett. 2008, 10, 2645–2648. [Google Scholar] [CrossRef]
- Bensari, A.; Zaveri, N.T. Titanium(IV) chloride-mediated ortho-acylation of phenols and naphthols. Synthesis 2003, 2003, 267–271. [Google Scholar]
- Anderson, K.W.; Tepe, J.J. Trifluoromethanesulfonic acid catalyzed Friedel–Crafts acylation of aromatics with β-lactams. Tetrahedron 2002, 58, 8475–8481. [Google Scholar] [CrossRef]
- Sarvari, M.H.; Sharghi, H. Simple and improved procedure for the regioselective acylation of aromatic ethers with carboxylic acids on the surface of graphite in the presence of methanesulfonic acid. Synthesis 2004, 2165–2168. [Google Scholar]
- Cook, D. The interaction of Friedel-Crafts catalysts with organic molecules I. The CH3COCl:AlCl3 system. Can. J. Chem. 1959, 37, 48–53. [Google Scholar] [CrossRef]
- Reifenrath, W.G.; Bertelli, D.J.; Micklus, M.J.; Fries, D.S. Stereochemistry of Friedel-Crafts addition of phthalylaspartic anhydride to benzene. Tetrahedron Lett. 1976, 17, 1959–1962. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, G.; Wang, X.; Wu, T.; Pan, X.; Chan, A.S.C.; Yang, T.K. The synthesis of l-(+)-homophenylalanine hydrochloride. Tetrahedron-Asymmetry 2000, 11, 2309–2314. [Google Scholar] [CrossRef]
- Nordlander, J.E.; Payne, M.J.; Njoroge, F.G.; Vishwanath, V.M.; Han, G.R.; Laikos, G.D.; Balk, M.A. A short enantiospecific synthesis of 2-amino-6,7-dihydroxy-1,2,3,4-tetrahydronaphthalene (ADTN). J. Org. Chem. 1985, 50, 3619–3622. [Google Scholar] [CrossRef]
- Horn, A.S.; Kazemier, H.G.; Dijkstra, D. Brain levels and metabolism of the dopaminergic agonist 2-amino-6,7-dihydroxytetrahydronaphthalene after administration of various prodrugs. J. Med. Chem. 1982, 25, 993–996. [Google Scholar]
- Griesbeck, A.G.; Heckroth, H. A simple approach to β-amino acids by acylation of arenes with N-acyl aspartic anhydrides. Synlett 1997, 1997, 1243–1244. [Google Scholar] [CrossRef]
- Buckley, T.F., III; Rapoport, H. α-Amino acids as chiral educts for asymmetric products. Amino acylation with N-acylamino acids. J. Am. Chem. Soc. 1981, 103, 6157–6163. [Google Scholar] [CrossRef]
- Ariyoshi, Y.; Yamatani, T.; Uchiyama, N.; Sato, N. The convenient preparation of L-aspartic anhydride hydrochloride and hydrobromide. Bull. Chem. Soc. Jpn. 1972, 45, 2208–2209. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Oh, P.C.; Shukor, S.R.A. Sustainable biocatalytic synthesis of l-homophenylalanine as pharmaceutical drug precursor. Biotechnol. Adv. 2009, 27, 286–296. [Google Scholar] [CrossRef]
- Wiechert, K. Neuere Methoden der präparativen organischen Chemie II 2. Verwendung von Fluorwasserstoff für organisch-chemische Reaktionen. Angew. Chem. 1943, 56, 333–342. (In German) [Google Scholar] [CrossRef]
- Baasner, B.; Klauke, E. Reactions in anhydrous hydrogen fluoride. J. Fluor. Chem. 1982, 19, 553–564. [Google Scholar] [CrossRef]
- Bednaiek, M.A. Amino acids with aryl-keto function in their side chains. J. Peptide Res. 1998, 52, 195–200. [Google Scholar] [CrossRef]
- Effenberger, F.; Epple, G. Catalytic Friedel-Crafts acylation of aromatic compounds. Angew. Chem. Int. Ed. Engl. 1972, 11, 300–301. [Google Scholar]
- Hwang, J.P.; Prakash, G.K.S.; Olah, G.A. Trifluoromethanesulfonic acid catalyzed novel Friedel–Crafts acylation of aromatics with methyl benzoate. Tetrahedron 2000, 56, 7199–7203. [Google Scholar] [CrossRef]
- Hashimoto, M.; Takahashi, M. Effective Friedel-Crafts acylations of O- and C-arylglycosides with triflic acid. Heterocycles 2009, 77, 227–231. [Google Scholar] [CrossRef]
- Muto, Y.; Murai, Y.; Sakihama, Y.; Hashidoko, Y.; Hashimoto, M. Effective Friedel-Crafts acylation of biotin acid chloride in trifluoromethanesulfonic acid. Biosci. Biotechnol. Biochem. 2012, 76, 2162–2164. [Google Scholar] [CrossRef]
- Murashige, R.; Hayashi, Y.; Hashimoto, M. Asymmetric and efficient synthesis of homophenylalanine derivatives via Friedel–Crafts reaction with trifluoromethanesulfonic acid. Tetrahedron Lett. 2008, 49, 6566–6568. [Google Scholar] [CrossRef]
- Svete, J.; Stanovnik, B.; Tiŝler, M. The synthesis of azatryptophane derivatives. J. Heterocycl. Chem. 1994, 31, 1259–1266. [Google Scholar] [CrossRef]
- Murashige, R.; Murai, Y.; Hatanaka, Y.; Hashimoto, M. Effective synthesis of optically active trifluoromethyldiazirinyl homophenylalanine and aroylalanine derivatives with the Friedel-Crafts reaction in triflic acid. Biosci. Biotechnol. Biochem. 2009, 73, 1377–1380. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hatanaka, Y. Recent progress in diazirine-based photoaffinity labeling. Eur. J. Org. Chem. 2008, 2008, 2513–2523. [Google Scholar] [CrossRef]
- Dubinsky, L.; Krom, B.P.; Meijler, M.M. Diazirine based photoaffinity labeling. Bioorg. Med. Chem. 2012, 20, 554–570. [Google Scholar] [CrossRef]
- Moss, R.A.; Fedé, J.M.; Yan, S. SbF5-mediated reactions of oxafluorodiazirines. Org. Lett. 2001, 3, 2305–2308. [Google Scholar] [CrossRef]
- Nakashima, H.; Hashimoto, M.; Sadakane, Y.; Tomohiro, T.; Hatanaka, Y. Simple and versatile method for tagging phenyldiazirine photophores. J. Am. Chem. Soc. 2006, 128, 15092–15093. [Google Scholar] [CrossRef]
- Ambroise, Y.; Mioskowski, C.; Djéga-Mariadassou, G.; Rousseau, B. Consequences of affinity in heterogeneous catalytic reactions: Highly chemoselective hydrogenolysis of iodoarenes. J. Org. Chem. 2000, 65, 7183–7186. [Google Scholar] [CrossRef]
- Okumura, H.S.; Philmus, B.; Portmann, C.; Hemscheidt, T.K. Homotyrosine-containing cyanopeptolins 880 and 960 and anabaenopeptins 908 and 915 from Planktothrix agardhii CYA 126/8. J. Nat. Prod. 2008, 72, 172–176. [Google Scholar]
- Canesi, S.; Bouchu, D.; Ciufolini, M.A. Fully stereocontrolled total syntheses of (−)-cylindricine C and (−)-2-epicylindricine C: A departure in sulfonamide chemistry. Angew. Chem. Int. Ed. 2004, 43, 4336–4338. [Google Scholar] [CrossRef]
- Murashige, R.; Hayashi, Y.; Ohmori, S.; Torii, A.; Aizu, Y.; Muto, Y.; Murai, Y.; Oda, Y.; Hashimoto, M. Comparisons of O-acylation and Friedel–Crafts acylation of phenols and acyl chlorides and Fries rearrangement of phenyl esters in trifluoromethanesulfonic acid: Effective synthesis of optically active homotyrosines. Tetrahedron 2011, 67, 641–649. [Google Scholar] [CrossRef]
- Plaza, A.; Bewley, C.A. Largamides A−H, unusual cyclic peptides from the marine cyanobacterium Oscillatoria sp. J. Org. Chem. 2006, 71, 6898–6907. [Google Scholar] [CrossRef]
- Hashimoto, K.; Yoshioka, T.; Morita, C.; Sakai, M.; Okuno, T.; Shirahama, H. Synthesis of a photoaffinity-labeling analog of alternariolide (AM-toxin I), a host-specific phytotoxin. Chem. Lett. 1998, 27, 203–204. [Google Scholar]
- Ueno, T.; Nakashima, T.; Hayashi, Y.; Fukami, H. Isolation and structure of AM-toxin III, a host specific phytotoxic metabolite produced by Alternaria mali. Agr. Biol. Chem. 1975, 39, 2081–2082. [Google Scholar] [CrossRef]
- Murai, Y.; Hashidoko, Y.; Hashimoto, M. Novel synthesis of optically active bishomotyrosine derivatives using the Friedel-Crafts reaction in triflic acid. Biosci. Biotechnol. Biochem. 2011, 75, 352–354. [Google Scholar] [CrossRef]
- Wang, J.; Bauman, S.; Colman, R.F. Photoaffinity labeling of rat liver glutathione S-transferase, 4-4, by glutathionyl S-[4-(succinimidyl)-benzophenone]. Biochemistry 1998, 37, 15671–15679. [Google Scholar] [CrossRef]
- Abe, I.; Zheng, Y.; Prestwich, G.D. Photoaffinity labeling of oxidosqualene cyclase and squalene cyclase by a benzophenone-containing inhibitor. Biochemistry 1998, 37, 5779–5784. [Google Scholar] [CrossRef]
- Kauer, J.C.; Erickson-Viitanen, S.; Wolfe, H.R.; DeGrado, W.F. p-Benzoyl-L-phenylalanine, a new photoreactive amino acid. J. Biol. Chem. 1986, 261, 10695–10700. [Google Scholar]
- Dorman, G.; Olszewski, J.D.; Prestwich, G.D. Synthesis of highly tritiated 4-benzoyl-L-phenylalanine, a photoactivatable amino acid. J. Org. Chem. 1995, 60, 2292–2297. [Google Scholar] [CrossRef]
- Murai, Y.; Wang, L.; Muto, Y.; Sakihama, Y.; Hashidoko, Y.; Hatanaka, Y.; Hashimoto, M. Simple and stereocontrolled preparation of benzoylated phenylalanine using Friedel–Crafts reaction in trifluoromethanesulfonic acid for photoaffinity labeling. Heterocycles 2013, 87, 2119–2126. [Google Scholar] [CrossRef]
- Murai, Y.; Wang, L.; Masuda, K.; Sakihama, Y.; Hashidoko, Y.; Hatanaka, Y.; Hashimoto, M. Rapid and controllable hydrogen/deuterium exchange on aromatic rings of α-amino acids and peptides. Eur. J. Org. Chem. 2013, 2013, 5111–5116. [Google Scholar] [CrossRef]
- Wang, L.; Murai, Y.; Yoshida, T.; Okamoto, M.; Masuda, K.; Sakihama, Y.; Hashidoko, Y.; Hatanaka, Y.; Hashimoto, M. Hydrogen-deuterium exchange of cross-linkable α-amino acid derivatives in deuterated triflic acid. Biosci. Biotechnol. Biochem. in press. 2014; in press. [Google Scholar]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, L.; Murai, Y.; Yoshida, T.; Okamoto, M.; Tachrim, Z.P.; Hashidoko, Y.; Hashimoto, M. Utilization of Acidic α-Amino Acids as Acyl Donors: An Effective Stereo-Controllable Synthesis of Aryl-Keto α-Amino Acids and Their Derivatives. Molecules 2014, 19, 6349-6367. https://doi.org/10.3390/molecules19056349
Wang L, Murai Y, Yoshida T, Okamoto M, Tachrim ZP, Hashidoko Y, Hashimoto M. Utilization of Acidic α-Amino Acids as Acyl Donors: An Effective Stereo-Controllable Synthesis of Aryl-Keto α-Amino Acids and Their Derivatives. Molecules. 2014; 19(5):6349-6367. https://doi.org/10.3390/molecules19056349
Chicago/Turabian StyleWang, Lei, Yuta Murai, Takuma Yoshida, Masashi Okamoto, Zetryana Puteri Tachrim, Yasuyuki Hashidoko, and Makoto Hashimoto. 2014. "Utilization of Acidic α-Amino Acids as Acyl Donors: An Effective Stereo-Controllable Synthesis of Aryl-Keto α-Amino Acids and Their Derivatives" Molecules 19, no. 5: 6349-6367. https://doi.org/10.3390/molecules19056349