Complexity and Uniqueness of the Aromatic Profile of Smoked and Unsmoked Herreño Cheese
Abstract
:1. Introdution
2. Results and Discussion
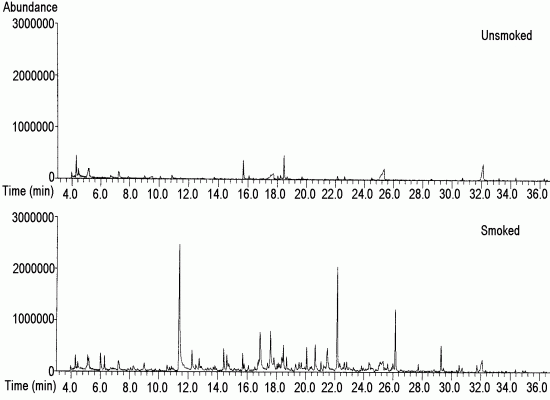
2.1. Characterization of the Unsmoked and of the Smoked Herreño Cheese


2.1.1. Acids, Alcohols, Esters and Lactones
| Nº | Compounds (Molecular Weight) | Bp | U | A | B |
|---|---|---|---|---|---|
| Acids | |||||
| Linear | |||||
| 1 | Acetic acid (60) * | 43 | 18.09 ± 8.32 a | 32.97 ± 7.13 a | 31.60 ± 4.75 a |
| 2 | Butanoic acid (88) * | 60 | 25.99 ± 2.82 b | 14.11 ± 2.56 a | 15.83 ± 1.22 a |
| 3 | Hexanoic acid (caproic acid) (116) * | 60 | 56.21 ± 4.80 b | 35.93 ± 3.88 a | 41.75 ± 5.36 a |
| 4 | Heptanoic acid (130) * | 60 | 1.24 ± 0.17 a | 1.77 ± 0.32 a | 1.70 ± 0.05 a |
| 5 | Octanoic acid (caprilic acid) (144) * | 60 | 43.27 ± 0.93 a | 35.94 ± 4.44 a | 40.85 ± 4.08 a |
| 6 | Nonanoic acid (158) * | 60 | 1.30 ± 0.14 a | 1.09 ± 0.10 a | 1.03 ± 0.21 a |
| Acids | |||||
| 7 | Decanoic acid (capric acid) (172) * | 60 | 28.55 ± 3.34 b | 17.31 ± 2.67 a | 21.27 ± 3.99 ab |
| 8 | Dodecanoic acid (lauric acid) (200) * | 73 | 0.97 ± 0.07 b | 0.55 ± 0.22 a | 0.50 ± 0.01 a |
| 9 | Hexadecanoic acid (palmitic acid) (256) * | 73 | - | 0.09 ± 0.03 a | 0.16 ± 0.05 a |
| Branched | |||||
| 10 | 2-Ethylhexanoic acid (144) | 88 | 0.52 ± 0.00 b | 0.35 ± 0.01 a | 0.31 ± 0.04 a |
| Aromatic | |||||
| 11 | Benzoic acid (122) | 105 | 10.94 ± 0.12 b | 4.44 ± 1.12 a | 5.03 ± 1.34 a |
| Alcohols | |||||
| Aliphatic | |||||
| 12 | Ethanol (46) * | 45 | 79.86 ± 0.24 b | 43.52 ± 6.46 a | 45.12 ± 1.64 a |
| 13 | 3-Methyl-1-butanol (88) (or isomer) | 55 | 2.74 ± 0.55 | - | - |
| 14 | 1-Pentanol (amilol) (86) | 42 | 1.16 ± 0.00 | - | - |
| Aromatic | |||||
| 15 | Benzenemethanol (108) * | - | 6.64 ± 0.76 a | 7.08 ± 1.21 a | |
| Esters | |||||
| Aliphatic | |||||
| 16 | Methyl acetate (74) | 43 | - | 8.48 ± 0.26 a | 10.86 ± 0.79 b |
| 17 | Methyl propenoate (86) | 55 | - | 1.88 ± 0.27 a | 2.91 ± 0.61 a |
| 18 | Methyl hydroxyacetate (90) | 58 | - | 2.83 ± 0.41 a | 1.95 ± 0.33 a |
| 19 | Methyl-2-butenoate (100) | 69 | - | 3.88 ± 0.02 a | 3.98 ± 0.84 a |
| 20 | Ethyl decanoate (200) * | 88 | 0.17 ± 0.01 a | 0.18 ± 0.01 a | 0.21 ± 0.03 a |
| 21 | 1-Methylethyl hexadecanoate (298) | 43 | - | 0.27 ± 0.08 a | 0.25 ± 0.24 a |
| Aromatic | |||||
| 24 | Methyl 4-methylbenzoate (150) | 119 | - | 1.49 ± 0.34 a | 1.48 ± 0.11 a |
| Lactones | |||||
| γ-Lactones | |||||
| 25 | 2(3H)-Dihydrofuranone (γ-butyrolactone) (86) | 42 | - | 5.52 ± 0.64 a | 5.13 ± 0.67 a |
| 26 | 2(5H)-Furanone (γ-crotonolactone) (84) * | 55 | - | 13.67 ± 1.93a | 11.48 ± 1.12 a |
| 27 | 5-Methyl-2(5H)furanone (98) * | 55 | - | 3.80 ± 0.32 a | 3.74 ± 0.39 a |
| 28 | 3-Methyl-2(5H)furanone (98) * | 41 | - | 8.47 ± 0.66 a | 8.45 ± 0.55 a |
| 29 | 5-Methyl-5-ethyldihydro-2(3H)-furanone (128) | 99 | - | 2.88 ± 0.42 a | 2.53 ± 0.11 a |
| 30 | γ-Dodecalactone (198) * | 85 | - | 0.31 ± 0.02 a | 0.34 ± 0.06 a |
| δ-Lactones | |||||
| 31 | 3,6-Dimethyl-2H-pyran-2-one (124) | 68 | - | 2.06 ± 0.30 a | 1.83 ± 0.18 a |
| 32 | 3-Hydroxy-2-methyl-4H-pyran-4-one (maltol) (126) * | 126 | - | 3.30 ± 0.67 a | 2.60 ± 0.46 a |
| 33 | δ-Octalactone (148) | 99 | 0.62 ± 0.04 a | 0.67 ± 0.07 a | 0.83 ± 0.10 a |
| 34 | δ-Decalactone (170) * | 99 | 1.56 ± 0.09 a | 1.62 ± 0.13 a | 1.86 ± 0.06 a |
| 35 | δ-Dodecalactone (198) * | 99 | 0.49 ± 0.03 a | 0.50 ± 0.09 a | 0.52 ± 0.02 a |
| Nº | Compounds (Molecular Weight) | Bp | U | A | B |
|---|---|---|---|---|---|
| Aldehydes | |||||
| Aliphatic saturated | |||||
| 36 | Pentanal (86) | 44 | 0.69 ± 0.07 a | 0.48 ± 0.04 a | 0.54 ± 0.13 a |
| 37 | Hexanal (100) * | 56 | 2.33 ± 0.18 b | 1.08 ± 0.14 a | 1.09 ± 0.13 a |
| 38 | Heptanal (114) | 70 | 0.17 ± 0.01 a | 0.26 ± 0.06 a | 0.24 ± 0.03 a |
| 39 | Nonanal (142) * | 57 | 2.51 ± 0.25 a | 2.50 ± 0.29 a | 3.18 ± 0.19 a |
| 40 | Decanal (156) | 43 | 0.33 ± 0.04 | - | - |
| 41 | Dodecanal (miristaldehyde) (184) | 57 | 0.29 ± 0.01 a | 0.28 ± 0.01 a | 0.28 ± 0.03 a |
| 42 | Tridecanal (268) | 57 | - | 0.10 ± 0.01 a | 0.14 ± 0.06 a |
| 43 | Tetradecanal (212) | 57 | 0.22 ± 0.01 a | 0.24 ± 0.04 a | 0.24 ± 0.05 a |
| Aliphatic unsaturated | |||||
| 44 | 2-Butenal (crotonaldehyde) (70) | 70 | 0.41 ± 0.04 a | 57.18 ± 1.83 b | 64.60 ± 16.91 b |
| 45 | 2-Methyl-2-butenal (or isomer) (84) | 84 | - | 1.84 ± 0.22 a | 1.81 ± 0.29 a |
| 46 | 2-Pentenal (84) | 55 | 0.56 ± 0.00 a | 3.95 ± 0.22 b | 4.53 ± 1.15 b |
| 47 | 2-Nonenal (140) | 41 | 0.20 ± 0.02 | - | - |
| Aromatic | |||||
| 48 | Benzaldehyde (106) * | 105 | 2.04 ± 0.30 a | 29.33 ± 3.31 b | 33.04 ± 5.13 b |
| 49 | Benzeneacetaldehyde (phenylacetaldehyde) (120) * | 91 | 1.13 ± 0.03 a | 2.71 ± 0.54 b | 3.07 ± 0.39 b |
| 50 | 2-Hydroxy-benzaldehyde (salicylaldehyde) (122) | 122 | - | 9.11 ± 1.63 a | 10.75 ± 2.00 a |
| 51 | Methylbenzaldehyde (120) | 119 | - | 9.28 ± 1.42 a | 10.53 ± 1.72 a |
| 52 | Methylbenzaldehyde (120) | 119 | - | 3.77 ± 0.76 a | 4.12 ± 0.43 a |
| 53 | 3-Hydroxy-4-methylbenzaldehyde (136) | 136 | - | 3.41 ± 0.64 a | 3.41 ± 0.55 a |
| 54 | 4-Hydroxy-3-methoxybenzaldehyde (vanillin) (152) * | 152 | - | 0.92 ± 0.09 a | 0.82 ± 0.21 a |
| Ketones and diketones | |||||
| Ketones | |||||
| Aliphatic | |||||
| 55 | 2-Propanone (58) * | 43 | 27.89 ± 0.38 a | 22.92 ± 6.68 a | 25.49 ± 4.74 a |
| 56 | 2-Butanone (72) * | 43 | 7.28 ± 1.59 a | 4.05 ± 0.64 a | 4.91 ± 1.34 a |
| 57 | 2-Pentanone (86) * | 43 | 3.09 ± 0.07 a | 6.29 ± 0.52 b | 6.93 ± 1.42 b |
| 58 | 4-Methyl-3-pentanone (100) | 57 | 0.42 ± 0.02 a | 2.08 ± 0.10 b | 2.40 ± 0.66 b |
| 59 | 3-Penten-2-one (84) | 69 | - | 8.97 ± 0.90 a | 8.86 ± 1.42 a |
| 60 | 2-Heptanone (114) * | 43 | 5.95 ± 0.34 a | 9.38 ± 0.10 b | 9.33 ± 1.35 b |
| 61 | 3,3-Dimethyl-2-butanone (100) | 57 | 0.30 ± 0.02 a | 22.48 ± 1.30 b | 23.14 ± 3.97 b |
| 62 | 2-Nonanone (142) * | 58 | 3.37± 0.05 a | 7.76 ± 0.61 b | 7.77 ± 1.37 b |
| 63 | 2-Undecanone (170) | 58 | 0.33 ± 0.03 a | 0.46 ± 0.03 b | 0.50 ± 0.06 b |
| Oxygenated aliphatic | |||||
| 64 | 1-Hydroxy-2-propanone (74) | 43 | 2.94 ± 0.28 a | 51.81 ± 7.23 b | 48.59 ± 2.39 b |
| 65 | 3-Hydroxy-2-butanone (acetoin) (88) * | 45 | 48.84± 8.81 a | 39.80 ± 16.25 a | 32.64 ± 0.24 a |
| 66 | 1-(Acetyloxy)-2-propanone (116) * | 43 | 0.83 ± 0.05 a | 59.52 ± 1.33 b | 59.15 ± 9.49 b |
| Cyclic | |||||
| 67 | Cyclopentanone (84) *b | 55 | 0.97 ± 0.02 a | 4.96 ± 0.84 b | 6.57 ± 1.00 b |
| 68 | Cyclohexanone (98) * | 55 | 0.28 ± 0.00 a | 3.69 ± 0.57 b | 4.66 ± 0.67 b |
| 69 | 2-Methylcyclopentanone (98) | 69 | 0.14 ± 0.00 a | 1.58 ± 0.09 b | 1.47 ± 0.25 b |
| 70 | 3-Methyl-2-cyclopenten-1-one (96) * | 96 | 1.98 ± 0.01 a | 43.49 ± 7.12 b | 47.70 ± 6.44 b |
| 71 | 2-Cyclohexen-1-one (96) | 68 | - | 4.90 ± 0.82 a | 5.31 ± 1.29 a |
| 72 | Dimethylcyclopent-2-en-1-one (110) | 67 | 0.54 ± 0.01 a | 6.84 ± 1.28 b | 8.12 ± 0.93 b |
| 73 | Dimethylcyclopent-2-en-1-one (110) | 95 | - | 7.57 ± 0.15 a | 6.78 ± 0.53 a |
| 74 | 2-Methyl-2-cyclopenten-1-one (96) * | 96 | 0.72 ± 0.02 a | 3.43 ± 0.20 b | 3.37 ± 0.55 b |
| 75 | Dimethylcyclopent-2-en-1-one (110) | 95 | 0.45 ± 0.04 a | 11.94 ± 1.26 b | 14.64 ± 2.71 b |
| 76 | 3-Methyl-2-cyclohexen-1-one (110) | 82 | - | 3.85 ± 0.45 a | 4.33 ± 0.67 a |
| 77 | Trimethyl-2-cyclopenten-1-one (124) | 109 | - | 9.84 ± 0.86 a | 9.36 ± 1.01 a |
| 78 | Dimethylcyclopent-2-en-1-one (110) | 67 | 1.22 ± 0.03 a | 40.61 ± 3.46 b | 41.45 ± 7.27 b |
| 79 | Trimethyl-2-cyclopenten-1-one (124) | 109 | 0.47 ± 0.01 a | 15.75 ± 1.46 b | 16.69 ± 2.65 b |
| Aromatic | |||||
| 80 | 1-(Methylphenyl)-ethanone (134) (or isomer) | 119 | - | 4.58 ± 0.46 a | 4.23 ± 0.63 a |
| 81 | 2,3-Dihydro-1H-inden-1-one (indanone) (132) | 132 | - | 6.91 ± 1.25 a | 5.34 ± 0.90 a |
| 82 | 3-Methyl-1-indanone (146) | 131 | - | 2.33 ± 0.27 a | 1.77 ± 0.28 a |
| 83 | 7-Methyl-1-indanone (146) | 117 | - | 1.28 ± 0.16 a | 0.98 ± 0.16 a |
| 84 | 4-Methyl-1-indanone (146) | 117 | - | 0.62 ± 0.12 a | 0.50 ± 0.12 a |
| 85 | 1-(2,6-Dihydroxy-4-methoxyphenyl)-ethanone (acetovanillone) (182) * | 167 | - | 1.69 ± 0.33 a | 1.17 ± 0.21 a |
| Diketones | |||||
| Linear | |||||
| 86 | 2,3-Butanedione (diacetyl) (86) * | 43 | 7.70 ± 4.19 a | 6.19 ± 0.75 a | 7.91 ± 0.94 a |
| 87 | 2,3-Pentanedione (100) | 43 | - | 4.53 ± 0.27 a | 5.13 ± 1.04 a |
| 88 | 2,5-Hexanedione (114) * | 43 | - | 6.16 ± 0.36 a | 6.32 ± 1.36 a |
| Cyclic | |||||
| 89 | Cyclopent-2-en-1,4-dione (96) | 96 | - | 20.43 ± 3.14 a | 23.86 ± 3.27 a |
| 90 | 3-Methyl-1,2-cyclopentanedione (cyclotene) (112) * | 112 | - | 17.12 ± 3.42 a | 12.98 ± 2.36 a |
| 91 | 4-Ethyl-cyclopentan-1,2-dione (126) | 97 | - | 9.18 ± 0.52 a | 9.26 ± 1.64 a |
| Nº | Compounds (Molecular Weight) | Bp | U | A | B |
|---|---|---|---|---|---|
| Hydrocarbons | |||||
| Aliphatic | |||||
| 92 | 4-Methyl-2-pentene (84) | 69 | - | 8.97 ± 0.90 a | 8.86 ± 1.42 a |
| 93 | 2-Heptene (98) | 57 | 1.93 ± 0.34 a | 2.02 ± 0.34 a | 2.59 ± 0.34 a |
| 94 | Nonane (128) * | 43 | 0.40 ± 0.02 a | 0.59 ± 0.06 b | 0.79 ± 0.07 b |
| 95 | cis-3,7-Dimethyl-2-octene (140) (or isomer) | 70 | 1.42 ± 0.12 a | 1.14 ± 0.20 a | 1.16 ± 0.26 a |
| 96 | Branched hydrocarbon | 57 | 11.57 ± 2.82 a | 14.52 ± 3.75 a | 12.07 ± 2.59 a |
| 97 | Linear hydrocarbon | 57 | 1.38 ± 0.29 a | 2.95 ± 0.16 b | 2.56 ± 0.06 b |
| 98 | Linear hydrocarbon | 57 | 2.70 ± 0.15 a | 3.87 ± 0.34 b | 3.34 ± 0.32 b |
| 99 | Linear hydrocarbon | 57 | 0.80 ± 0.11 a | 1.01 ± 0.11 a | 1.20 ± 0.20 a |
| 100 | Undecane (156) | 43 | 0.43 ± 0.08 a | 0.68 ± 0.02 ab | 0.76 ± 0.17 b |
| 101 | Branched hydrocarbon | 57 | 0.47 ± 0.07 a | 0.68 ± 0.08 b | 0.72 ± 0.09 b |
| 102 | Dodecane (170) * | 57 | 0.52 ± 0.07 a | 0.72 ± 0.08 b | 0.76 ± 0.12 b |
| 103 | Tridecane (184) | 43 | 0.28 ± 0.01 a | 0.52 ± 0.02 b | 0.54 ± 0.06 b |
| 104 | Tetradecane (198) * | 57 | 2.88 ± 0.02 b | 1.25 ± 0.10 a | 1.34 ± 0.08 a |
| 105 | Pentadecane (212) * | 57 | 0.32 ± 0.07 ab | 0.26 ± 0.01 a | 0.36 ± 0.01 b |
| 106 | Hexadecane (226) * | 57 | 0.20 ± 0.01 a | 0.23 ± 0.03 a | 0.25 ± 0.04 a |
| 107 | 3,7,11,15-Tetramethyl-2-hexadecene (phytene) (280) | 70 | 0.23 ± 0.00 a | 0.20 ± 0.12 a | 0.21 ± 0.07 a |
| Monoaromatic | |||||
| 108 | Benzene (78) | 78 | 0.50 ± 0.05 a | 6.79 ± 1.63 b | 8.72 ± 0.87 b |
| 109 | Toluene (92) * | 91 | 12.52 ± 2.03 a | 19.75 ± 3.62 b | 20.45 ± 4.67 b |
| 110 | Dimethylbenzene (106) | 91 | 0.95 ± 0.16 a | 4.03 ± 0.66 b | 4.33 ± 0.37 b |
| 111 | Dimethylbenzene (106) | 91 | 2.89 ± 0.07 a | 8.23 ± 1.37 b | 10.78 ± 2.09 b |
| 112 | Styrene (104) * | 104 | 1.75 ± 0.16 a | 13.22 ± 3.67 b | 12.25 ± 2.06 b |
| 113 | Methylmethylethylbenzene (isomer) (134) | 119 | 2.27 ± 0.03 a | 2.20 ± 0.36 a | 2.60 ± 0.06 a |
| Polyaromatic | |||||
| 114 | 1H-Indene (116) | 130 | 0.35 ± 0.08 a | 9.13 ± 1.60 b | 11.42 ± 0.63 b |
| 115 | 1-Methyl-1H-indene (130) | 130 | - | 2.74 ± 0.69 a | 2.80 ± 0.41 a |
| 116 | 2-Methyl-1H-indene (130) | 130 | - | 1.60 ± 0.20 a | 1.80 ± 0.11 a |
| 117 | Azulene (128) | 128 | 0.09 ± 0.05 a | 2.49 ± 0.69 b | 2.47 ± 0.24 b |
| 118 | Naphtalene (128) * | 128 | 1.84 ± 0.56 a | 37.49 ± 7.91 b | 36.87 ± 2.67 b |
| 119 | Decahydronaphthalene (138) | 138 | - | 1.06 ± 0.19 a | 0.86 ± 0.15 a |
| 120 | 2-Methylnaphthalene (142) * | 142 | 0.17 ± 0.06 a | 3.09 ± 0.11 b | 3.00 ± 0.29 b |
| 121 | 1-Methylnaphthalene (142) * | 142 | 0.10 ± 0.06 a | 2.57 ± 0.45 b | 2.09 ± 0.15 b |
| 122 | 1,1'-Biphenyl (154) | 154 | - | 1.07 ± 0.15 a | 0.88 ± 0.06 a |
| 123 | Dimethylnaphthalene (156) | 156 | - | 0.33 ± 0.05 a | 0.28 ± 0.03 a |
| 124 | Dimethylnaphthalene (156) | 156 | - | 0.40 ± 0.05 b | 0.29 ± 0.01 a |
| 125 | Dimethylnaphthalene (156) | 156 | - | 0.13 ± 0.02 a | 0.10 ± 0.01 a |
| 126 | Acenaphthylene (152) * | 152 | - | 0.99 ± 0.08 b | 0.73 ± 0.06 a |
| Terpenes | |||||
| 127 | δ-3-Carene (136) | 93 | 1.23 ± 0.13 a | 0.89 ± 0.20 a | 0.86 ± 0.09 a |
| 128 | α-Thujene (136) | 93 | 0.73 ± 0.07 a | 0.69 ± 0.18 a | 0.70 ± 0.14 a |
| 129 | α-Pinene (136) * | 93 | 34.91 ± 1.79 a | 25.68 ± 5.49 a | 24.71 ± 2.58 a |
| 130 | Camphene (136) * | 93 | 1.65 ± 0.00 a | 1.20 ± 0.20 a | 1.09 ± 0.21 a |
| 131 | β-Terpinene (136) * | 93 | 0.51 ± 0.06 a | 0.59 ± 0.08 a | 0.70 ± 0.05 a |
| 132 | β-Pinene (136) * | 93 | 2.39 ± 0.06 a | 4.86 ± 0.68 b | 4.79 ± 0.64 b |
| 133 | Camphane (138) | 95 | 29.33 ± 0.71 b | 18.94 ± 3.08 a | 20.81 ± 1.28 a |
| 134 | α-Phellandrene (136) * | 93 | 3.78 ± 0.14 a | 2.00 ± 0.20 a | 2.41 ± 0.18 a |
| 135 | Menth-1-ene (138) | 138 | 0.17 ± 0.00 a | 0.98 ± 0.23 b | 0.89 ± 0.03 b |
| 136 | trans-Carane (138) or trans-p-Menth-1-ene (138) | 95 | 0.41 ± 0.01 | - | - |
| 137 | α-Limonene (136) * | 68 | 2.68 ± 0.12 a | 5.78 ± 0.58 b | 6.71 ± 1.62 b |
| 138 | α-Fenchene (136) | 93 | 0.12 ± 0.02 a | 0.73 ± 0.17 b | 0.76 ± 0.05 b |
| 139 | Alloocimene (136) | 121 | - | 0.87 ± 0.38 a | 1.10 ± 0.17 a |
| 140 | α-Terpinolene (136) | 136 | - | 1.73 ± 0.24 a | 1.70 ± 0.41 a |
| 141 | α-Terpinene (136) * | 136 | - | 0.26 ± 0.04 b | 0.15 ± 0.02 a |
| 142 | β-Camphor (152) *b | 152 | - | 0.32 ± 0.05 a | 0.46 ± 0.11 a |
| Sesquiterpenes | |||||
| 143 | α-Copaene (or isomer) (204) | 161 | 0.44 ± 0.01 b | 0.32 ± 0.02 a | 0.39 ± 0.05 ab |
| 144 | Cyperene (or isomer) (204) | 204 | 0.21 ± 0.00 a | 0.28 ± 0.02 ab | 0.32 ± 0.06 b |
| 145 | α-Gurjunene (or isomer) (204) | 161 | 0.06 ± 0.00 a | 0.11 ± 0.00 b | 0.13 ± 0.03 b |
| 146 | Junipene (or isomer) (204) | 161 | 0.09 ± 0.01 a | 0.11 ± 0.02 a | 0.11 ± 0.04 a |
| 147 | trans-Caryophyllene (204) * | 93 | 1.08 ± 0.02 a | 1.06 ± 0.05 a | 1.06 ± 0.37 a |
| 148 | Alloaromadendrene (or isomer) (204) | 161 | 0.07 ± 0.00 a | 0.12 ± 0.01 b | 0.14 ± 0.03 b |
| 149 | α-Humulene (or isomer) (204) | 93 | 0.21 ± 0.00 a | 0.21 ± 0.01 a | 0.22 ± 0.00 a |
| 150 | Widdrene (or isomer) (204) | 119 | 0.16 ± 0.05 a | 0.17 ± 0.02 a | 0.20 ± 0.01 a |
| 151 | Calarene (or isomer) (204) | 161 | 0.09 ± 0.02 a | 0.13 ± 0.02 a | 0.15 ± 0.03 a |
| 152 | β-Cadinene (or isomer) (204) | 204 | 0.05 ± 0.00 a | 0.04 ± 0.00 a | 0.05 ± 0.01 a |
| 153 | Valencene (or isomer) (204) | 161 | 0.07 ± 0.00 a | 0.08 ± 0.01 a | 0.09 ± 0.02 a |
| Nº | Compounds (Molecular Weight) | Bp | U | A | B |
|---|---|---|---|---|---|
| Nitrogenated derivatives | |||||
| Nitrile derivatives | |||||
| 154 | Benzonitrile (103) | 103 | 1.66 ± 0.25 a | 53.89 ± 5.98 b | 63.61 ± 9.01 b |
| 155 | 3-Methylbenzonitrile (117) | 117 | 0.04 ± 0.02 a | 1.50 ± 0.25 b | 1.73 ± 0.20 b |
| 156 | Benzeneacetonitrile (117) | 117 | - | 2.40 ± 0.38 a | 2.34 ± 0.64 a |
| 157 | Benzenepropanenitrile (131) | 91 | - | 2.14 ± 0.38 a | 1.76 ± 0.25 a |
| Pyridine and pyrazine derivatives and others | |||||
| 158 | Pyrazine (80) | 80 | 0.60 ± 0.04 a | 6.79 ± 0.58 b | 7.92 ± 1.51 b |
| 159 | Pyridine (79) * | 79 | 2.16 ± 0.06 a | 29.84 ± 0.43 b | 34.88 ± 5.80 b |
| 160 | 4-Methylpyridine (93) | 93 | 0.71 ± 0.20 a | 11.39 ± 1.54 b | 13.61 ± 1.78 b |
| 161 | Methylpyrazine (94) | 94 | 0.73 ± 0.03 a | 12.97 ± 1.02 b | 15.13 ± 3.00 b |
| 162 | 2-Methylpyridine (93) | 93 | 0.61 ± 0.05 a | 19.92 ± 2.50 b | 22.55 ± 3.31 b |
| 163 | 2,6-Dimethylpyridine (107) * | 107 | - | 2.15 ± 0.36 a | 2.08 ± 0.31 a |
| 164 | 2-Ethylpyridine (107) * | 106 | 0.19 ± 0.01 a | 4.40 ± 1.00 b | 5.64 ± 0.96 b |
| 165 | 2,4-Dimethylpyridine (107) * | 107 | - | 6.12 ± 1.06 a | 7.76 ± 1.27 a |
| 166 | 3,4-Dimethylpyridine (107) | 107 | - | 2.49 ± 0.36 a | 2.70 ± 0.32 a |
| 167 | 3-Ethylpyridine (107) | 92 | - | 2.49 ± 0.45 a | 2.88 ± 0.42 a |
| 168 | 3-Methoxypyridine (109) * | 109 | - | 15.14 ± 0.64 a | 15.81 ± 2.07 a |
| 169 | 2-Ethyl-6-methylpyridine (121) | 120 | - | 1.86 ± 0.30 a | 1.86 ± 0.22 a |
| 170 | 5-Ethyl-2-methylpyridine (121) * | 120 | - | 2.02 ± 0.24 a | 2.42 ± 0.48 a |
| 171 | 2-Methoxy-3-methylpyrazine (124) | 124 | - | 7.05 ± 0.53 a | 6.58 ± 1.25 a |
| 172 | β-Nicotyrine (158) | 158 | 0.53 ± 0.11 a | 0.61 ± 0.12 a | 0.60 ± 0.01 a |
| Nº | Compounds (Molecular Weight) | Bp | U | A | B |
|---|---|---|---|---|---|
| Phenol derivatives | |||||
| Phenol | |||||
| 173 | Phenol (94) * | 94 | - | 173.11 ± 18.44 a | 149.26 ± 21.62 a |
| 174 | 2-Methylphenol (108) | 107 | - | 46.52 ± 7.71 a | 39.09 ± 6.76 a |
| 175 | 3-Methylphenol (108) * + | 107 | - | 79.36 ±15.04 a | 64.64 ± 10.73 a |
| 4-Methylphenol (108) * | |||||
| 176 | 2,6-Dimethylphenol (122) * | 122 | - | 13.47 ± 1.23 a | 12.22 ± 1.90 a |
| 177 | 2-Ethylphenol (122) * + | 107 | - | 6.32 ± 0.90 a | 5.37 ± 0.79 a |
| 2-Propylphenol (136) * | |||||
| 178 | 2,4-Dimethylphenol (122) * | 122 | - | 12.32 ± 2.13 a | 10.00 ± 2.05 a |
| 179 | 2,5-Dimethylphenol (122) * | 122 | - | 11.95 ± 1.78 a | 9.88 ± 1.84 a |
| 180 | Dimethylphenol (122) | 122 | - | 6.51 ± 1.17 a | 5.53 ± 0.60 a |
| 181 | Dimethylphenol (122) | 122 | - | 12.64 ± 2.34 a | 13.09 ± 2.51 a |
| 182 | Dimethylphenol (122) | 122 | - | 3.73 ± 0.59 a | 2.98 ± 0.55 a |
| 183 | 2-(1-Methylethyl)-phenol (136) | 121 | - | 2.28 ± 0.13 a | 1.97 ± 0.45 a |
| 184 | 2,4,6-Trimethylphenol (136) * | 121 | - | 1.73 ± 0.19 a | 1.44 ± 0.27 a |
| 185 | 2,3,5-Trimethylphenol (136) * | 121 | - | 1.80 ± 0.19 a | 1.49 ± 0.24 a |
| 186 | Diethylphenol (150) | 135 | - | 1.93 ± 0.23 a | 1.73 ± 0.17 a |
| 187 | 3,4,5-Trimethylphenol (136) * | 121 | - | 1.41 ± 0.22 a | 1.25 ± 0.23 a |
| 188 | 2,6-Bis(1,1-dimethylethyl)-4-methylphenol (BHT) (220) * | 205 | - | 0.29 ± 0.04 a | 0.25 ± 0.00 a |
| 189 | 2,4,5-Tri-sec-buthylphenol (262) | 203 | - | 0.32 ± 0.02 a | 0.34 ± 0.06 a |
| Methoxyphenol derivatives | |||||
| 190 | 2-Methoxyphenol (guaiacol) (124) * | 109 | - | 250.50 ± 24.70 a | 231.94 ± 33.36 a |
| 191 | 2-Methoxy-4-methylphenol (4-methylguaiacol) (138) * | 123 | - | 107.65 ± 8.76 a | 88.25 ± 13.92 a |
| 192 | 4-Ethyl- 2-methoxyphenol (4-ethylguaiacol) (152) * | 137 | - | 70.36 ± 9.01 a | 55.65 ± 8.93 a |
| 193 | 4-Vinyl-2-methoxyphenol (4-vinylguaiacol) (150) * | 135 | - | 11.53 ± 0.95 a | 9.76 ± 2.10 a |
| 194 | 4-(2-Propenyl)-2-methoxyphenol (eugenol) (164) * | 164 | - | 4.00 ± 0.62 a | 3.25 ± 0.66 a |
| 195 | 2-Methoxy-4-propylphenol (4-propylguayacol) (166) * | 137 | - | 5.86 ± 0.67 a | 4.66 ± 0.79 a |
| 196 | 4-(1-Propenyl)-2-methoxyphenol (isoeugenol isomer) (164) * | 164 | - | 1.50 ± 0.25 a | 1.16 ± 0.30 a |
| 197 | 4-(1-Propenyl)-2-methoxyphenol (isoeugenol isomer) (164) * | 164 | - | 3.06 ± 0.42 a | 2.15 ± 0.26 a |
| Dimethoxyphenol derivatives | |||||
| 198 | Dimethoxyphenol (154) (isomer) | 154 | - | 0.54 ± 0.01 a | 0.52 ± 0.07 a |
| 199 | 2,6-Dimethoxyphenol (syringol) (154) * | 154 | - | 11.18 ± 0.22 a | 11.31 ± 1.93 a |
| Ethers | |||||
| Alkyl-aryl ethers | |||||
| 200 | Methoxybenzene (108) | 108 | - | 3.41 ± 0.38 a | 3.91 ± 0.49 a |
| 201 | 4-Methyl-1-methoxybenzene (122) | 122 | - | 7.01 ± 2.03 a | 8.82 ± 1.37 a |
| 202 | Ethoxy-benzene (122) | 94 | - | 3.01 ± 0.76 a | 3.49 ± 0.60 a |
| 203 | 1,1-Dimethylethoxybenzene (150) | 94 | - | 1.10 ± 0.08 a | 0.96 ± 0.12 a |
| 204 | 1,2-Dimethoxybenzene (veratrol) (138) * | 123 | - | 5.39 ± 0.67 a | 5.42 ± 1.26 a |
| 205 | 1,4-Dimethoxybenzene (138) * | 123 | - | 12.69 ± 0.73 a | 11.81 ± 0.96 a |
| 206 | Dimethoxytoluene (152) (isomer) | 152 | 0.13 ± 0.01 a | 12.71 ± 2.14 b | 11.49 ± 2.57 b |
| 207 | 3,4-Dimethoxytoluene (152) (or isomer) | 152 | 0.01 ± 0.00 a | 3.01 ± 0.34 b | 2.62 ± 0.27 b |
| 208 | 5-Methyl-1,2,3-trimethoxybenzene (182) | 182 | - | 1.14 ± 0.04 b | 0.85 ± 0.17 a |
| 209 | Trimethoxybenzene (168) (isomer) | 168 | - | 3.39 ± 0.39 a | 2.94 ± 0.05 a |
| Furan derivatives | |||||
| Benzofuran derivatives | |||||
| 210 | Benzofuran (118) | 118 | 1.52 ± 0.09 a | 30.72 ± 4.44 b | 36.01 ± 5.76 b |
| 211 | Methyl-benzofuran (132) (isomer) | 131 | 0.12 ± 0.06 a | 3.10 ± 0.66 b | 3.47 ± 0.50 b |
| 212 | Methyl-benzofuran (132) (isomer) | 131 | 0.35 ± 0.14 a | 8.51 ± 2.04 b | 9.42 ± 1.05 b |
| 213 | Methyl-benzofuran (132) (isomer) | 131 | 0.45 ± 0.17 a | 10.81 ± 2.11 b | 11.34 ± 1.86 b |
| 214 | Dimethyl-benzofuran (146) (isomer) | 146 | 0.09 ± 0.03 a | 2.18 ± 0.24 b | 2.52 ± 0.20 b |
| 215 | Dimethyl-benzofuran (146) (isomer) | 146 | 0.16 ± 0.05 a | 3.83 ± 0.95 b | 3.81 ± 0.17 b |
| 216 | Methoxy-benzofuran (148) | 148 | - | 1.25 ± 0.08 b | 1.06 ± 0.09 a |
| Others | |||||
| 217 | 2-Methylfuran (82) | 82 | - | 3.06 ± 0.15 a | 6.36 ± 1.36 b |
| 218 | 3-Furaldehyde (96) | 95 | - | 3.35 ± 0.70 a | 3.71 ± 0.86 a |
| 219 | 2-Furancarboxaldehyde (96) * | 96 | - | 698.19 ± 105.95 a | 725.90 ± 148.68 a |
| 220 | 2-Furanmethanol (98) * | 98 | 0.50 ± 0.16 a | 47.23 ± 5.12 b | 43.03 ± 7.10 b |
| 221 | 1-2-(Furanyl)-ethanone (2-acetylfuran) (110) * | 95 | 2.60 ± 0.09 a | 81.61 ± 8.70 b | 90.94 ± 12.94 b |
| 222 | 5-Methyl-2-furancarboxaldehyde (110) * | 110 | 0.96 ± 0.10 a | 153.68 ± 20.11 b | 144.53 ± 35.56 b |
| 223 | Methyl furancarboxylate (126) | 95 | - | 38.37 ± 3.51 a | 41.74 ± 7.58 a |
| 224 | 1-(2-Furanyl)-1-propanone (124) | 95 | - | 11.41 ± 0.50 a | 11.86 ± 1.92 a |
| 225 | 2-Butyltetrahydrofuran (128) | 71 | - | 31.85 ± 2.25 a | 30.46 ± 4.81 a |
| 226 | 2-(2-Propenyl)-furan (108) | 79 | - | 3.40 ± 0.49 a | 3.77 ± 0.83 a |
| 227 | 5-Hydroxymethyl-2-furancarboxaldehyde (126) * | 97 | - | 9.10 ± 0.65 a | 9.96 ± 2.26 a |
| 228 | 2-(2-Furanylmethyl)-5-methylfuran (162) | 162 | - | 0.92 ± 0.10 a | 0.91 ± 0.01 a |
2.1.2. Aldehydes, Ketones and Diketones
2.1.3. Hydrocarbons, Terpenes and Sesquiterpenes
2.1.4. Nitrogenated Derivatives
2.1.5. Phenol Derivatives and Ethers
2.2. Comparison of the Unsmoked Herreño Cheese with Other Types of Goat Cheeses
2.3. Comparison of the Smoked Herreño Cheese with Other Smoked Cheeses
2.4. Influence of the Position in the Smokehouse on the Volatile Profile of Smoked Herreño Cheese
3. Material and Methods
3.1. Cheeses and Smoking Process
3.2. Generation of the Headspace and Extraction of its Components by SPME
3.3. Study of the Extracted Compounds by Gas Chromatography/Mass Spectrometry
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barbieri, G.; Bolzoni, L.; Careri, M.; Mangia, A.; Parolari, G.; Spagnoli, S.; Virgili, R. Study of the volatile fraction of Parmesan cheese. J. Agric. Food Chem. 1994, 42, 1170–1176. [Google Scholar]
- Kubícková, J.; Grosch, W. Evaluation of potent odorants of Camembert cheese by dilution and concentration techniques. Int. Dairy J. 1997, 7, 65–70. [Google Scholar]
- Rychlik, M.; Bosset, J.O. Flavour and off-flavour compounds of Swiss Gruyère cheese. Evaluation of potent odorants. Int. Dairy J. 2001, 11, 895–901. [Google Scholar] [CrossRef]
- Pérès, C.; Viallon, C.; Berdagué, J.L. Solid-phase microextraction-mass spectrometry: A new approach to the rapid characterization of cheeses. Anal. Chem. 2001, 73, 1030–1036. [Google Scholar] [CrossRef]
- Pinho, O.; Ferreira, I.M.P.L.V.O.; Casal, S.; Fernandes, J.O.; Oliveira, M.B.P.P.; Ferreira, M.A. Method optimization for analysis of the volatile fraction of ewe cheese by solid-phase microextraction. Chromatographia 2001, 53, S390–S393. [Google Scholar] [CrossRef]
- Pinho, O.; Ferreira, I.M.P.L.V.O.; Ferreira, M.A. Solid-phase microextraction in combination with GC/MS for quantification of the major volatile free fatty acids in Ewe cheese. Anal. Chem. 2002, 74, 5199–5204. [Google Scholar] [CrossRef]
- Vítová, E.; Loupancová, B.; Štoudková, H.; Zemanová, J. Application of SPME-GC method for analysis of the aroma of white surface mould cheeses. J. Food Nutr. Res. 2007, 46, 84–90. [Google Scholar]
- Delgado, F.J.; González-Crespo, J.; Cava, R.; García-Parra, J.; Ramírez, R. Characterisation by SPME-GC-MS of the volatile profile of a Spanish soft cheese P.D.O. Torta del Casar during ripening. Food Chem. 2010, 118, 182–189. [Google Scholar] [CrossRef]
- Ferreira, I.M.P.L.V.O.; Pinho, O.; Sampaio, P. Volatile fraction of DOP “Castelo Branco” cheese: Influence of breed. Food Chem. 2009, 112, 1053–1059. [Google Scholar]
- Wolf, I.V.; Perotti, M.C.; Bernal, S.M.; Zalazar, C.A. Study of the chemical composition, proteolysis, lipolysis and volatile compounds profile of commercial Reggianito Argentino cheese: Characterization of Reggianito Argentino cheese. Food Res. Int. 2010, 43, 1204–1211. [Google Scholar] [CrossRef]
- Guillén, M.D.; Abascal, B. Nature and distribution of the volatile components in the different regions of an artisanal ripened sheep cheese. J. Dairy Res. 2012, 79, 102–109. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ibargoitia, M.L.; Sopelana, P.; Palencia, G.; Fresno, M. Components detected by means of solid-phase microextraction and gas chromatography/mass spectrometry in the headspace of artisan fresh goat cheese smoked by traditional methods. J. Dairy Sci. 2004, 87, 284–299. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ibargoitia, M.L. Volatile Components of aqueous liquid smokes from vitis vinifera L shoots and fagus sylvatica L Wood. J. Sci. Food Agric. 1996, 72, 104–110. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ibargoitia, M.L. New components with potential antioxidant and organoleptic properties, detected for the first time in liquid smoke flavoring preparations. J. Agric. Food Chem. 1998, 46, 1276–1285. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ibargoitia, M.L.; Sopelana, P.; Palencia, G. Components detected by headspace-solid phase microextraction in artisanal fresh goat’s cheese smoked using dry prickly pear (Opuntia ficus indica). Lait 2004, 84, 385–397. [Google Scholar] [CrossRef]
- McSweeny, P.L.H.; Sousa, M.J. Biochemical pathways for the production of flavour compounds in cheeses during ripening: A review. Lait 2000, 80, 293–324. [Google Scholar] [CrossRef]
- Curioni, P.M.G.; Bosset, J.O. Key odorants in various cheese types as determined by gas chromatography-olfactometry. Int. Dairy J. 2002, 12, 959–984. [Google Scholar] [CrossRef]
- Bosset, J.O.; Gubler, M.; Bütikofer, U.; Gauch, R. Mono-, di- and trimethyl benzene in frozen cheese samples: Natural metabolites or environmental pollutants. Mitt. Lebensmittelunters. Hyg. 2000, 91, 287–299. [Google Scholar]
- Molimard, P.; Spinnler, H.E. Compounds involved in the flavor of surface mold-ripened cheese: Origins and properties. J. Dairy Sci. 1996, 79, 169–184. [Google Scholar] [CrossRef]
- Grova, N.; Feidt, C.; Crepineau, C.; Laurent, C.; Lafargue, P.E.; Hachimi, A.; Rychen, G. Detection of polycyclic aromatic hydrocarbon levels in milk collected near potential contamination sources. J. Agric. Food Chem. 2002, 50, 4640–4642. [Google Scholar] [CrossRef]
- Gonzalez, A.G.; Rodriguez Diaz, F. Alkaloids in plants of the Canary Island. VII. Nicotiana glauca and N. paniculata. Anales Real. Soc. Espan. Fis. Quim. 1962, 58, 431–436. [Google Scholar]
- Delgado, F.J.; González-Crespo, J.; Cava, R.; Ramírez, R. Formation of the aroma of a raw goat milk cheese during maturation analysed by SPME-GC-MS. Food Chem. 2011, 129, 1156–1163. [Google Scholar] [CrossRef]
- Hayaloglu, A.A.; Tolu, C.; Yasar, K.; Sahingil, D. Volatiles and sensory evaluation of goat milk cheese Gokceada as affected by goat breeds (Gokceada and Turkish Saanen) and starter culture systems during ripening. J. Dairy. Sci. 2013, 96, 2765–2780. [Google Scholar] [CrossRef]
- Chiofalo, B.; Zumbo, A.; Costa, R.; Liotta, L.; Mondello, L.; Dugo, P.; Chiofalo, V. Characterization of Maltese goat milk cheese flavour using SPME-GC/MS. S. Afri. J. Ani. Sci. 2004, 34, 176–180. [Google Scholar]
- Bontinis, T.G.; Mallatou, H.; Pappa, E.C.; Massouras, T.; Alichanidis, E. Study of proteolysis, lipolysis and volatile profile of a traditional Greek goat cheese (Xinotyri) during ripening. Small Rumin. Res. 2012, 105, 193–201. [Google Scholar] [CrossRef]
- Massouras, T.; Pappa, E.C.; Mallatou, H. Headspace analysis of volatile flavour compounds of Teleme cheese made from sheep and goat milk. Int. J. Dairy Technol. 2006, 59, 250–256. [Google Scholar] [CrossRef]
- Attaie, R. Quantification of volatile compounds in goat milk Jack cheese using static headspace gas chromatography. J. Dairy Sci. 2009, 92, 2435–2443. [Google Scholar] [CrossRef]
- Condurso, C.; Verzera, A.; Romeo, V.; Ziino, M.; Conte, F. Solid-phase microextraction and gas chromatography mass spectrometry analysis of dairy product volatiles for the determination of shelf-life. Int. Dairy J. 2008, 18, 819–825. [Google Scholar] [CrossRef]
- Mondello, L.; Costa, R.; Tranchida, P.Q.; Chiofalo, B.; Zumbo, A.; Dugo, P.; Dugo, G. Determination of flavor components in Sicilian goat cheese by automated HS-SPME-GC. Flavour Frag. J. 2005, 20, 659–665. [Google Scholar] [CrossRef]
- Carunchia-Whetstine, M.E.; Karagul-Yuceer, Y.; Avsar, Y.K.; Drake, M.A. Identification and quantification of character aroma components in fresh Chevre-style goat cheese. J. Food Sci. 2003, 68, 2441–2447. [Google Scholar] [CrossRef]
- Castillo, I.; Calvo, M.V.; Alonso, L.; Juarez, M.; Fontecha, J. Changes in lipolysis and volatile fraction of a goat cheese manufactured employing a hygienized rennet paste and a defined strain starter. Food Chem. 2007, 100, 590–598. [Google Scholar] [CrossRef]
- Serhan, M.; Linder, M.; Hosri, C.; Fanni, J. Changes in proteolysis and volatile fraction during ripening of Darifayeh, a Lebanese artisanal raw goat's milk cheese. Small Rumin. Res. 2010, 90, 75–82. [Google Scholar] [CrossRef]
- Sablé, S.; Letellier, F.; Cottenceau, G. An analysis of the volatile flavour compounds in a soft raw goat milk cheese. Biotechnol. Lett. 1997, 19, 143–145. [Google Scholar] [CrossRef]
- Fernández-García, E.; Imhof, M.; Schlichtherle-Cerny, H.; Bosset, J.O.; Nuñez, M. Terpenoids and benzenoids in La Serena cheese made at different seasons of the year with a Cynara cardunculus extract as coagulant. Int. Dairy J. 2008, 18, 147–157. [Google Scholar]
- Frank, D.C.; Owen, C.M.; Patterson, J. Solid phase microextraction (SPME) combined with gas-chromatography and olfactometry-mass spectrometry for characterization of cheese aroma compounds. LWT - Food Sci. Technol. 2004, 37, 139–154. [Google Scholar] [CrossRef]
- Poveda, J.M.; Sánchez-Palomo, E.; Pérez-Coello, M.S.; Cabezas, L. Volatile composition, olfactometry profile and sensory evaluation of semi-hard Spanish goat cheeses. Dairy Sci. Technol. 2008, 88, 355–367. [Google Scholar] [CrossRef]
- Majcher, M.A.; Goderska, K.; Pikul, J.; Jeleń, H.H. Changes in volatile, sensory and microbial profiles during preparation of smoked ewe cheese. J. Sci. Food Agric. 2011, 91, 1416–1423. [Google Scholar] [CrossRef]
- Guillén, M.D.; Palencia, G.; Ibargoitia, M.L.; Fresno, M.; Sopelana, P. Contamination of cheese by PAHs in traditional smoking. Influence of the position in the smokehouse on the contamination level of smoked cheese. J. Dairy Sci. 2011, 94, 1679–1690. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Palencia, G.; Ibargoitia, M.L.; Fresno, M.; Sopelana, P.; Guillén, M.D. Complexity and Uniqueness of the Aromatic Profile of Smoked and Unsmoked Herreño Cheese. Molecules 2014, 19, 7937-7958. https://doi.org/10.3390/molecules19067937
Palencia G, Ibargoitia ML, Fresno M, Sopelana P, Guillén MD. Complexity and Uniqueness of the Aromatic Profile of Smoked and Unsmoked Herreño Cheese. Molecules. 2014; 19(6):7937-7958. https://doi.org/10.3390/molecules19067937
Chicago/Turabian StylePalencia, Gemma, Maria Luisa Ibargoitia, Maria Fresno, Patricia Sopelana, and Maria Dolores Guillén. 2014. "Complexity and Uniqueness of the Aromatic Profile of Smoked and Unsmoked Herreño Cheese" Molecules 19, no. 6: 7937-7958. https://doi.org/10.3390/molecules19067937