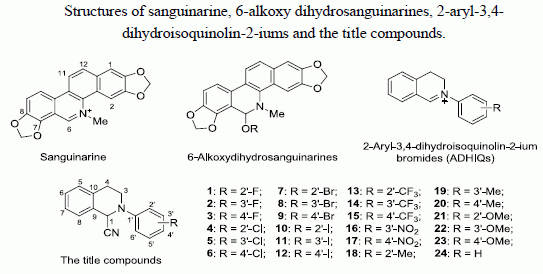
3.2. General Procedure for the Synthesis of Compounds 1–24
To the solution of 2-aryl-3,4-dihydroisoquinolin-2-ium bromine (0.5 mmol) in ethanol or 95% ethanol in water (ca. 20 mL), KCN (76 mg, 1.17 mmol) was added. The reaction solution was stirred at room temperature or 0 °C for 30 min, and then diluted with CHCl3 or CH2Cl2 (80 mL). The resulting solution was washed with water (3 × 30 mL), and dried over anhydrous sodium sulfate. The solvent was removed under a reduced pressure to provide crude product as an oil or solid. The crude product was subjected to column chromatography over silica gel eluting with the mixture of petroleum ether and ethyl acetate (10:1) to afford 1–24 as solids or liquids.
2-(2-Fluorophenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (
1): white solid; yield: 74%; m.p. 88–89 °C; IR (KBr)
υmax cm
−1: 2224 (w, C≡N), 1142 (s, C−N), 1228 (s, C−F); UV (MeOH): λ
max (lg
ε) 237(4.09) nm;
1H-NMR (CDCl
3)
δ: 7.29–7.33 (1H, m), 7.25–7.27 (2H, m), 7.21–7.23 (2H, m), 7.16–7.19 (1H, m), 7.10–7.12 (2H, m), 5.48 (1H, s, H-1), 3.60–3.51 (2H, m, H-3), 3.22 (1H, ddd,
J = 17.0, 11.0, 7.0 Hz, H-4a), 2.93 (1H, d-like, H-4b);
13C-NMR (CDCl
3)
δ: 156.1 (d,
J = 245.0 Hz, C-2'), 136.9 (d,
J = 9.2 Hz, C-1'), 134.0, 129.6, 129.3, 128.7, 127.1, 126.8, 125.0 (d,
J = 4.4 Hz), 121.5 (d,
J = 2.0 Hz), 117.5 (C≡N), 116.4 (d,
J = 20.2 Hz), 53.9 (C-1), 44.8 (C-3), 28.6 (C-4); positive ESI-MS
m/z: 226 [M−CN]
+, 253 [M+H]
+, 275 [M+Na]
+. The IR,
1H-NMR and
13C-NMR data are agreement with the literature data [
17].
Crystallographic data and X-ray structure analysis of
1. A colorless crystal (0.50 × 0.28 × 0.24) of
1 was grown by slow evaporation in petroleum ether-AcOEt solution. Diffraction intensity data were acquired with a CCD diffractometer with graphite-monochromated Mo
Ká radiation (λ = 0.71073 Å). Crystal data for
1: C
16H
13FN
2,
Mr = 252.28, Orthorhombic, space group Pbca, Z = 8,
a = 7.4686(7),
b = 14.9034(14),
c = 23.239(2) Å,
α = 90.00°,
β = 90.00°,
γ = 90.00°,
V = 2586.7(4) Å
3,
T = 296(2) K,
μ (Mo Ká) = 0.087 mm
−1, 17836 reflections measured, 2402 independent reflections (
Rint = 0.0252),
S = 0.993. The final
R1 values were 0.0363 and
wR2 = 0.1182 (
I > 2
σ (
I)). The final
R1 values were 0.0472 and
wR2 = 0.1324 (for all data). Crystallographic data for
1 have been deposited with the Cambridge Crystallographic Data Centre (deposition number CCDC 986701). Copies of the data can be obtained, free of charge, on application to the Director, CCDC, 12 Union Road, Cambridge CB21EZ, UK (fax: +44(0)-1223–336033 or e-mail:
[email protected]. ac.uk).
2-(3-Fluorophenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (2): white solid; yield: 83%; m.p. 94–95 °C; IR (KBr) υmax cm−1: 2219 (w, C≡N, 1146 (s, C−N), 1174 (s, C−F); UV (MeOH): λmax (lg ε) 246 (4.12) nm; 1H-NMR (CDCl3) δ: 7.28–7.37 (5H, m), 6.85 (1H, dd, J = 8.5, 2.5 Hz), 6.79 (1H, dt, J = 11.5, 2.5 Hz), 6.73 (1H, td, J = 8.5, 2.5 Hz), 5.54 (1H, s, H-1), 3.78–3.83 (1H, m, H-3a), 3.53 (1H, ddd, J = 12.5, 10.5, 4.0 Hz, H-3b), 3.19 (1H, ddd, J = 16.5, 10.5, 6.0 Hz, H-4a), 3.05 (1H, dt, J = 16.0, 4.0 Hz, H-4b); 13C-NMR (CDCl3) δ: 163.8 (d, J = 243.6 Hz, C-3'), 149.8 (d, J = 9.6 Hz, C-1'), 134.6, 130.8 (d, J = 9.9 Hz), 129.3, 129.0, 127.1, 117.5 (C≡N), 112.2 (d, J = 2.6 Hz), 108.1 (d, J = 21.1 Hz), 104.1 (d, J = 25.1 Hz), 52.3 (C-1), 44.1 (C-3), 28.4 (C-4); positive ESI-MS m/z: 226 [M−CN]+, 253 [M+H]+, 275 [M+Na]+. HR-MS [M+Na]+: Calcd. for C16H13FN2Na+, 275.0960, found 275.0969.
2-(4-Fluorophenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (
3): white solid; yield: 88%; m.p. 124–125 °C; IR (KBr)
υmax cm
−1: 2226 (w, C≡N), 1142 (s, C−N), 1244 (s, C−F); UV (MeOH): λ
max (lg
ε) 240 (3.97) nm;
1H-NMR (CDCl
3)
δ: 7.23–7.34 (4H, m), 7.08 (2H, s), 7.07 (2H, d,
J = 2.5 Hz), 5.40 (1H, s, H-1), 3.63 (1H, q-like, H-3a), 3.46 (1H, td,
J = 11.0, 4.0 Hz, H-3b), 3.17 (1H, ddd,
J = 17.0, 11.0, 6.5 Hz, H-4a), 2.96 (1H, dt,
J = 16.0, 3.5 Hz, H-4b);
13C-NMR (CDCl
3)
δ: 158.6 (d,
J = 240.4 Hz, C-4'), 145.1 (d,
J = 2.5 Hz, C-1'), 134.3, 129.5, 129.4, 128.8, 127.1, 126.9, 120.5 (d,
J = 10.5 Hz), 117.4 (C≡N), 116.2 (d,
J = 22.4 Hz), 54.8 (C-1), 44.8 (C-3), 28.6 (C-4); positive ESI-MS
m/z: 226 [M−CN]
+, 253 [M+H]
+, 275 [M+Na]
+. The IR,
1H-NMR and
13C-NMR data are agreement with the literature data [
17].
2-(2-Chlorophenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (
4): white sheet-like crystals; yield: 87%; m.p. 115–116 °C; IR (KBr)
υmax cm
−1: 2224 (w, C≡N), 1142 (s, C−N); UV (MeOH): λ
max (lg
ε) 243 (3.92) nm;
1H-NMR (CDCl
3)
δ: 7.44 (1H, d-like,
J = 8.0 Hz), 7.30–7.35 (3H, m), 7.23–7.27 (3H, m), 7.13–7.16 (1H, m), 5.53 (1H, s, H-1), 3.62 (1H, td,
J = 12.0, 4.0 Hz, H-3a), 3.46 (1H, dd,
J = 12.0, 6.5 Hz, H-3b), 3.26 (1H, ddd,
J = 16.5, 11.5, 6.0 Hz, H-4a), 2.93 (1H, dd,
J = 16.5, 2.5 Hz, H-4b);
13C-NMR (CDCl
3)
δ: 145.9 (C-1'), 134.1, 130.7, 129.7, 128.7, 128.2, 127.1, 126.7, 126.1, 123.2, 117.4 (C≡N), 53.9 (C-1), 45.6 (C-3), 28.8 (C-4); positive ESI-MS
m/z: 242 [M−CN]
+, 269 [M+H]
+, 291[M+Na]
+. The IR,
1H-NMR and
13C-NMR are in agreement with the literature data [
17].
2-(3-Chlorophenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (
5): white powder; yield: 78%; m.p. 83–85 °C; IR (KBr)
υmax cm
−1: 2223 (w, C≡N), 1153 (s, C−N); UV (MeOH): λ
max (lg
ε) 251 (4.41) nm;
1H-NMR (CDCl
3)
δ: 7.23–7.32 (5H, m), 7.03 (1H, t-like), 6.92–6.97 (2H, m), 5.49 (1H, s, H-1), 3.72–3.76 (1H, m, H-3a), 3.48 (1H, ddd,
J = 12.5, 10.5, 4.5 Hz, H-3b), 3.12 (1H, ddd,
J = 16.0, 10.0, 5.5 Hz, H-4a), 2.97 (1H, dt,
J = 16.5, 4.0 Hz, H-4b);
13C-NMR (CDCl
3)
δ: 149.4 (C-1'), 135.4, 134.5, 130.6, 129.3, 129.2, 129.0, 127.1, 121.5, 117.5 (C≡N), 117.2, 115.0, 52.4 (C-1), 44.1 (C-3), 28.4 (C-4); positive ESI-MS
m/z: 242[M−CN]
+, 269.0[M+H]
+, 291[M+Na]
+. The IR,
1H-NMR and
13C-NMR data are agreement with the literature data [
17].
2-(4-Chlorophenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (
6): white powder; yield: 78%; m.p. 152–153 °C; IR (KBr)
υmax cm
−1: 2223 (w, C≡N), 1142 (s, C−N); UV (MeOH): λ
max (lg
ε) 253 (4.59) nm;
1H-NMR (CDCl
3)
δ: 7.31–7.35 (5H, m), 7.27–7.28 (1H, m), 7.02–7.05 (2H, m), 5.49 (1H, s, H-1), 3.74 (1H, ddd-like, H-3a), 3.50 (1H, ddd-like, H-3b), 3.18 (1H, ddd,
J = 16.0, 10.5, 6.0 H-4a), 3.00 (1H, dt,
J = 16.0, 3.5 Hz, H-4b);
13C-NMR (CDCl
3)
δ: 147.0 (C-1'), 134.4, 129.6, 129.4, 129.3, 128.9, 127.1, 127.0, 118.9, 117.5 (C≡N), 53.1 (C-1), 44.3 (C-3), 28.5 (C-4); positive ESI-MS
m/z: 242 [M−CN]
+. The IR,
1H-NMR and
13C-NMR data are agreement with the literature data [
17].
2-(2-Bromophenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (7): white powder; yield: 90%; m.p. 119–120 °C; IR (KBr) υmax cm−1: 2224 (w, C≡N); 1141 (s, C−N); UV (MeOH): λmax (lg ε) 240 (4.12) nm; 1H-NMR (CDCl3) δ: 7.62 (1H, dd, J = 7.5, 1.0 Hz), 7.40 (1H, td-like), 7.30–7.35 (2H, m), 7.23–7.27 (3H, m), 5.53 (1H, s, H-1), 3.64 (1H, dt, J = 12.0, 4.0 Hz, H-3a), 3.42 (1H, dd, J = 12.0, 6.5 Hz, H-3b), 3.27 (1H, ddd, J = 17.0, 11.5, 6.5 Hz, H-4a), 2.92 (1H, dd, J = 16.5, 3.0 Hz, H-4b); 13C-NMR (CDCl3) δ: 147.2 (C-1'), 134.2, 133.8, 129.7, 129.5, 128.9, 128.7, 127.1, 126.7, 123.8, 120.5, 117.3(C≡N), 54.3 (C-1), 45.8 (C-3), 28.9 (C-4); positive ESI-MS m/z: 286 [M−CN]+, 313 [M+H]+. HR-MS [M+Na]+: Calcd. for C16H13BrN2Na+, 335.0160, found 335.0155.
2-(3-Bromophenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (8): white sheet crystal; yield: 75%; m.p. 92–93 °C; IR (KBr) υmax cm−1: 2220 (w, C≡N), 1150 (s, C−N); UV (MeOH): λmax (lg ε) 252 (4.13) nm; 1H-NMR (CDCl3) δ: 7.31–7.37 (3H, m), 7.24–7.29 (3H, m), 7.15–7.17 (1H, m), 7.02–7.03 (1H, m), 5.53 (1H, s, H-1), 3.77–3.81 (1H, m, H-3a), 3.53 (1H, td, J = 11.5, 4.0 Hz, H-3b), 3.18 (1H, ddd, J = 16.0, 10.5, 6.0 Hz, H-4a), 3.02 (1H, d, J = 16.5 Hz, H-4b); 13C-NMR (CDCl3) δ: 149.5 (C-1'), 134.5, 130.9, 129.4, 129.2, 129.0, 127.1, 127.0, 124.4, 123.6, 120.1, 117.5 (C≡N), 115.4, 52.4 (C-1), 44.1 (C-3), 28.4 (C-4); positive ESI-MS m/z: 286[M−CN]+. HR-MS [M+Na]+: Calcd. for C16H13BrN2Na+, 335.0160, found 335.0154.
2-(4-Bromophenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (
9): white sheet crystal; yield: 72%; m.p. 156–157 °C; IR (KBr)
υmax cm
−1: 2222 (w, C≡N), 1138 (s, C−N); UV (MeOH): λ
max (lg
ε) 204 (4.70), 255 (4.48) nm;
1H-NMR (CDCl
3)
δ: 7.45 (2H, d-like,
J = 8.5 Hz), 7.28–7.34 (3H, m), 7.23–7.25 (1H, m), 6.95 (2H, d-like,
J = 9.0 Hz), 5.45 (1H, s, H-1), 3.69–3.74 (1H, m, H-3a), 3.46 (1H, ddd,
J = 12.5, 10.5, 4.0 Hz, H-3b), 3.17 (1H, ddd,
J = 16.5, 10.5, 6.0 Hz, H-4a), 2.98 (1H, dt,
J = 13.0, 3.5 Hz, H-4b);
13C-NMR (CDCl
3)
δ: 147.4 (C-1'), 134.4, 132.5, 129.4, 129.2, 129.0, 127.1, 127.0, 119.1, 117.5 (C≡N), 114.4, 52.9 (C-1), 44.3 (C-3), 28.4 (C-4); positive ESI-MS
m/z: 286 [M−CN]
+, 313 [M+H]
+. The IR,
1H-NMR and
13C-NMR data are agreement with the literature data [
17].
2-(2-Iodophenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (10): white powder; yield: 83%; m.p. 127–128 °C; IR (KBr) υmax cm−1: 2223 (w, C≡N), 1138 (s, C−N); UV (MeOH): λmax (lg ε) 204 (4.01) nm; 1H-NMR (CDCl3) δ: 7.90 (1H, d, J = 7.5 Hz), 7.42–7.45 (1H, t-like, J = 7.5 Hz), 7.31–7.34 (2H, m), 7.23–7.27 (3H, m), 6.95 (1H, t, J = 7.5 Hz, H-6'), 5.43 (1H, s, H-1), 3.63–3.68 (1H, t-like, J = 11.5 Hz, H-3a), 3.35–3.38 (1H, m, H-3b), 3.23–3.30 (1H, m, H-4a), 2.92 (1H, d, J = 16.5 Hz, H-4b); 13C-NMR (CDCl3) δ: 150.0 (C-1'), 140.1, 134.3, 129.8, 129.7, 129.5, 128.7, 127.6, 127.1, 126.7, 123.9, 117.3 (C≡N), 98.5 (C-2'), 55.1 (C-1), 46.2 (C-3), 29.1 (C-4); positive ESI-MS m/z: 334 [M−CN]+, 361 [M+H]+, 383 [M+Na]+. HR-MS [M+Na]+: Calcd. for C16H13IN2Na+, 383.0021, found 383.0015.
2-(3-Iodophenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (11): white powder; yield: 79%; m.p. 124–125 °C; IR (KBr) υmax cm−1: 2220 (w, C≡N), 1135 (s, C−N); UV (MeOH): λmax (lg ε) 252 (4.30) nm; 1H-NMR (CDCl3) δ: 7.39 (1H, s, H-2’), 7.29–7.33 (4H, m), 7.24–7.25 (1H, m), 7.01–7.08 (2H, m), 5.47 (1H, s, H-1), 3.72–3.76 (1H, m, H-3a), 3.45–3.51 (1H, m, H-3b), 3.14 (1H, ddd, J = 16.5, 10.5, 6.0 Hz, H-4a), 2.95 (1H, dt, J = 16.0, 4.0 Hz, H-4b); 13C-NMR (CDCl3) δ: 149.5 (C-1'), 134.5, 131.0, 130.6, 129.3, 129.2, 129.0, 127.1, 126.2, 117.5 (C≡N), 116.2, 95.3, 52.5 (C-1), 44.1 (C-3), 28.4 (C-4); positive ESI-MS m/z: 334 [M−CN]+, 383 [M+Na]+. HR-MS [M+Na]+: Calcd. for C16H13IN2Na+, 383.0021, found 383.0017.
2-(4-Iodophenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (12): white powder; yield: 88%; m.p. 145–147 °C; IR (KBr) υmax cm−1: 2221 (w, C≡N), 1140 (s, C−N); UV (MeOH): λmax (lg ε) 258 (4.39) nm; 1H-NMR (CDCl3) δ: 7.66 (2H, d-like, J = 9.0 Hz, H-3', H-5'), 7.31–7.34 (3H, m), 7.26–7.28 (1H, m), 6.86 (2H, d-like, J = 9.0 Hz, H-2', H-6'), 5.49 (1H, s, H-1), 3.73–3.78 (1H, m, H-3a), 3.47–3.52 (1H, m, H-3b), 3.17 (1H, ddd, J = 16.5, 10.5, 6.0 Hz, H-4a), 3.01 (1H, dt, J = 16.0, 4.0 Hz, H-4b); 13C-NMR (CDCl3) δ: 148.0, 138.4 (C-3', C-5'), 134.5, 129.4, 129.2, 129.0, 127.1, 127.0, 119.3, 117.5 (C≡N), 84.2, 52.5 (C-1), 44.1 (C-3), 28.4 (C-4); positive ESI-MS m/z: 334 [M−CN]+, 361 [M+H]+, 383 [M+Na]+. HR-MS [M+Na]+: Calcd. for C16H13IN2Na+, 383.0021, found 383.0014.
2-(2-(Trifluoromethyl)phenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (13): white powder; yield: 90%; m.p. 101–102 °C; IR (KBr) υmax cm−1: 2224 (w, C≡N), 1169 (s, C−N), 1312 (s, C−F); UV (MeOH): λmax (lg ε) 245 (4.05) nm; 1H-NMR (CDCl3) δ: 7.70 (2H, d, J = 8.0 Hz), 7.65 (1H, t, J = 7.5 Hz), 7.39 (1H, t, J = 7.5 Hz), 7.31 (1H, t, J = 7.5 Hz), 7.20–7.26 (3H, m), 5.13 (1H, s, H-1), 3.65–3.71 (1H, m, H-3a), 3.20–3.28 (2H, m, H-3b, H-4a), 2.87–2.91 (1H, m, H-4b); 13C-NMR (CDCl3) δ: 148.3, 134.1, 133.4, 129.8, 129.7, 128.6, 127.3 (q, J = 5.0 Hz), 126.9 (d, J = 7.5 Hz), 126.6, 126.4, 117.9 (C≡N), 56.2 (C-1), 47.2 (C-3), 28.9 (C-4); positive ESI-MS m/z: 276 [M−CN]+, 325 [M+Na]+. HR-MS [M+Na]+: Calcd. for C17H13F3N2Na+, 325.0929, found 325.0933.
2-(3-(Trifluoromethyl)phenyl)-1,2-dihydroisoquinoline-1-carbonitrile (14): white crystal; yield: 93%; m.p. 100–101 °C; IR (KBr) υmax cm−1: 2226 (w, C≡N), 1166 (s, C−N), 1327 (s, C−F); UV (MeOH): λmax (lg ε) 251 (4.64) nm; 1H-NMR (CDCl3) δ: 7.47 (1H, t, J = 8.0 Hz), 7.30–7.35 (3H, m), 7.22–7.27 (4H, m), 5.54 (1H, s, H-1), 3.79–3.83 (1H, m, H-3a), 3.54 (1H, td-like, J = 10.0, 4.0 Hz, H-3b), 3.17 (1H, ddd, J = 16.0, 10.0, 6.0 Hz, H-4a), 3.02 (1H, dt, J = 16.5, 4.0 Hz, H-4b); 13C-NMR (CDCl3) δ: 148.5, 134.4, 132.0 (d, J = 32.0 Hz), 130.2, 129.4, 129.1, 129.0, 119.8, 118.0, 117.4 (C≡N), 113.7, 52.4 (C-1), 44.2 (C-3), 28.4 (C-4); positive ESI-MS m/z: 276 [M−CN]+. HR-MS [M+Na]+: Calcd. for C17H13F3N2Na+, 325.0929, found 325.0939.
2-(4-(Trifluoromethyl)phenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (15): white powder; yield: 98%; m.p. 101–102 °C; IR (KBr) υmax cm−1: 2224 (w, C≡N), 1172 (s,C−N), 1326 (s, C−F); UV (MeOH): λmax (lg ε) 205 (4.40), 259 (4.32) nm; 1H-NMR (CDCl3) δ: 7.61 (2H, d, J = 8.5 Hz, H-3', H-5'), 7.26–7.37 (4H, m), 7.09 (2H, d, J = 8.5 Hz, H-2', H-6'), 5.58 (1H, s, H-1), 3.84–3.88 (1H, m, H-3a), 3.55–3.60 (1H, m, H-3b), 3.17 (1H, ddd, J = 16.0, 10.0, 6.0 Hz, H-4a), 3.06 (1H, dt, J = 16.0, 8.5, 4.5 Hz, H-4b); 13C-NMR (CDCl3) δ: 150.4 (C-1'), 134.6, 129.3, 129.1, 129.1, 127.2, 127.1, 126.9, 126.9, 117.5 (C≡N), 115.4, 51.3 (C-1), 44.0 (C-3), 28.3 (C-4); positive ESI-MS m/z: 276 [M−CN]+, 325 [M+Na]+. HR-MS [M+Na]+: Calcd. for C17H13F3N2Na+, 325.0929, found 325.0935.
2-(3-Nitrophenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (16): yellow powder; yield: 90%; m.p. 168–170 °C; IR (KBr) υmax cm−1: 2224 (w, C≡N), 1149 (s, C−N), 1579 (s, NO2); UV (MeOH): λmax (lg ε) 211 (4.13), 247 (4.31) nm; 1H-NMR (CDCl3) δ: 7.89 (1H, s, H-2'), 7.83 (1H, d, J = 8.0 Hz), 7.52 (1H, t, J = 8.0 Hz), 7.32–7.36 (4H, m), 7.26–7.29 (1H, m), 5.60 (1H, s, H-1), 3.85–3.89 (1H, m, H-3a), 3.57–3.62 (1H, m, H-3b), 3.19 (1H, ddd, J = 16.0, 10.0, 6.0 Hz, H-4a), 3.08 (1H, dt, J = 16.0, 4.0 Hz, H-4b); 13C-NMR (CDCl3) δ: 148.9 (C-1'), 134.4 (C-3'), 130.4, 129.3, 129.2, 128.8, 127.3, 127.1, 121.7, 117.2 (C≡N), 115.7, 111.0, 51.7 (C-1), 44.1 (C-3), 28.3 (C-4); positive ESI-MS m/z: 253 [M−CN]+, 302 [M+Na]+. HR-MS [M+Na]+: Calcd. for C16H13N3NaO2+, 302.0905, found 302.0900.
2-(4-Nitrophenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (17): yellow powder; yield: 93%; m.p. 102–104 °C; IR (KBr) υmax cm−1: 2361 (w, C≡N), 1162 (s, C−N), 1597 (s, NO2); UV (MeOH): λmax (lg ε) 368 (4.00) nm; 1H-NMR (CDCl3) δ: 8.16–8.18 (2H, d, J = 9.0 Hz, H-2', H-6'), 7.25–7.33 (4H, m), 6.94–6.98 (2H, d, J = 8.5 Hz, H-3', H-5'), 5.79 (1H, s, H-1), 3.77–3.81 (1H, m, H-3a), 3.50–3.56 (1H, m, H-3b), 3.38–3.44 (1H, m, H-4a), 2.97 (1H, dt, J = 15.0, 5.0 Hz, H-4b); 13C-NMR (CDCl3) δ: 153.6 (C-1'), 138.9 (C-4'), 135.7, 133.6, 129.0, 128.0, 126.5, 126.1, 126.0, 113.4 (C≡N), 112.3, 61.4 (C-1), 43.7 (C-3), 27.8 (C-4); positive ESI-MS m/z: 253 [M−CN]+. HR-MS [M+Na]+: Calcd. for C16H13N3NaO2+, 302.0905, found 302.0901.
2-(2-Methylphenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (18): yellow crystal; yield: 82%; m.p. 136–138 °C; IR (KBr) υmax cm−1: 2220 (w, C≡N), 1141 (s, C−N); UV (MeOH): λmax (lg ε) 237 (3.88) nm; 1H-NMR (CDCl3) δ: 7.22–7.31 (7H, m), 7.14–7.11 (1H, m), 5.06 (1H, s, H-1), 3.61 (1H, td, J = 11.4, 3.8 Hz, H-3a), 3.35–3.32 (1H, m, H-3b), 3.18 (1H, ddd, J = 16.8, 11.4, 6.2 Hz, H-4a), 2.92 (1H, d-like, J = 16.8 Hz, H-4b), 2.29 (3H, s); 13C-NMR (CDCl3) δ: 148.0 (C-1'), 134.5, 133.4, 131.2, 130.1, 129.7, 128.6, 127.2, 127.0, 126.6, 125.4, 122.0, 117.7 (C≡N), 55.6 (C-1), 44.9 (C-3), 28.7 (C-4); positive ESI-MS m/z: 222 [M−CN]+. HR-MS [M+Na]+: Calcd. for C17H16N2Na+, 271.1211, found 271.1207.
2-(3-Methylphenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (
19): yellow oil; yield: 75%; IR (KBr)
υmax cm
−1: 2224 (w, C≡N), 1030 (vs, C−N); UV (MeOH): λ
max (lg
ε) 247 (4.12) nm;
1H-NMR (CDCl
3)
δ: 7.28–7.20 (5H, m), 6.88–6.86 (2H, m), 6.82 (1H, d,
J = 7.5 Hz, H-4'), 5.49 (1H, s, H-1), 3.74 (1H, ddd,
J = 12.4, 6.0, 2.9 Hz, H-3a), 3.44 (1H, ddd,
J = 12.4, 10.9, 4.1 Hz, H-3b), 3.12 (1H, ddd,
J = 16.4, 10.9, 6.0 Hz, H-4a), 2.92 (1H, dt,
J = 16.4, 3.4 Hz, H-4b), 2.35 (3H, s);
13C-NMR (CDCl
3)
δ: 148.5 (C-1'), 139.4, 134.7, 129.7, 129.5, 129.4, 128.8, 127.1, 126.9, 122.8, 118.4, 117.9 (C≡N), 114.7, 53.3 (C-1), 44.2 (C-3), 28.6 (C-4); positive ESI-MS
m/z: 222[M−CN]
+. The IR,
1H-NMR and
13C-NMR data are agreement with the literatural data [
17].
2-(4-Methylphenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (
20): yellow solid; yield: 64%; m.p. 114–116 °C; IR (KBr)
υmax cm
−1: 2222 (w, C≡N), 1137 (s, C−N); UV (MeOH): λ
max (lg
ε) 245 (4.11) nm;
1H-NMR (CDCl
3)
δ: 7.30–7.21 (4H, m), 7.16 (2H, d,
J = 8.2 Hz, H-3', 5'), 7.00 (2H, d,
J = 8.4 Hz, H-2', 6'), 5.45 (1H, s, H-1), 3.70 (1H, ddd,
J = 12.4, 6.1, 2.3 Hz, H-3a), 3.44 (1H, ddd,
J = 12.4, 11.2, 4.0 Hz, H-3b), 3.15 (1H, ddd,
J = 16.7, 11.2, 6.1 Hz, H-4a), 2.94 (1H, dt,
J = 16.7, 3.2 Hz, H-4b), 2.31 (3H, s);
13C-NMR (CDCl
3)
δ: 146.3 (C-1'), 134.6, 131.8, 130.1 (C-3', 5'), 129.7, 129.4, 128.7, 127.1, 126.8, 118.3 (C-2', 6'), 117.7 (C≡N), 114.8, 54.1 (C-1), 44.4 (C-3), 28.6 (C-4); positive ESI-MS
m/z: 222 [M−CN]
+. The IR,
1H-NMR and
13C-NMR data are agreement with the literature data [
17].
2-(2-Methoxyphenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (
21): white solid; yield: 67%; m.p. 150–152°C; IR (KBr)
υmax cm
−1: 2223 (w, C≡N), 1144 (s, C−N), 1243 (vs, C-O-C); UV (MeOH): λ
max (lg
ε) 241 (3.84), 278 (3.48) nm;
1H-NMR (CDCl
3)
δ: 7.12–7.30 (6H, m), 7.02 (1H, td,
J = 8.0, 1.0 Hz,), 6.92 (1H, d,
J = 8.0 Hz), 5.74 (1H, s, H-1), 3.85 (3H, s), 3.52 (2H, dd,
J = 8.0 Hz, H-3), 3.20–3.26 (1H, m, H-4a), 2.92 (1H, dt,
J = 16.5, 2.5 Hz, H-4b);
13C-NMR (CDCl
3)
δ: 152.5 (C-2'), 137.8 (C-1'), 134.1, 129.9, 129.6, 128.5, 127.2, 126.6, 125.1, 121.5, 121.0, 117.8 (C≡N), 111.5, 55.6 (C-1), 53.2, 44.8 (C-3), 28.8 (C-4); positive ESI-MS
m/z: 238 [M−CN]
+, 265 [M+H]
+, 287 [M+Na]
+. The IR,
1H-NMR and
13C-NMR data are agreement with the literature data [
17].
2-(3-Methoxyphenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (
22): yellow oil; yield: 73%; IR (KBr)
υmax cm
−1: 2221(w, C≡N), 1165 (s, C−N), 1212 (vs, C-O-C)); UV (MeOH): λ
max (lg
ε) 245 (4.09) nm;
1H-NMR (CDCl
3)
δ: 7.21–7.31 (5H, m), 6.54–6.68 (3H, m), 5.51 (1H, s, H-1), 3.81 (3H, s), 3.74–3.77 (1H, m, H-3a), 3.43–3.49 (1H, m, H-3b), 3.10–3.16 (1H, m, H-4a), 2.95 (1H, d,
J = 16.5 Hz, H-4b);
13C-NMR (CDCl
3)
δ: 160.8 (C-3'), 149.7 (C-1'), 134.7, 130.4, 129.6, 129.4, 128.8, 127.1, 126.9, 117.8 (C≡N), 109.9, 106.6, 104.0, 55.4(C-1), 53.0, 44.2 (C-3), 28.5 (C-4); positive ESI-MS
m/z: 238 [M−CN]
+, 265 [M+H]
+. The IR,
1H-NMR and
13C-NMR data are agreement with the literature data [
17].
2-(4-Methoxyphenyl)-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (
23): yellow oil; yield: 78%; IR (KBr)
υmax cm
−1: 2222 (w, C≡N), 1136 (s, C−N), 1248 (vs, C-O-C); UV (MeOH): λ
max (lg
ε) 242 (4.05) nm;
1H-NMR (CDCl
3)
δ: 7.21–7.30 (4H, m), 7.08 (2H, d,
J = 9.0 Hz, H-3', H-5'), 6.91 (2H, d,
J = 9.0 Hz, H-2', H-6'), 5.36 (1H, s, H-1), 3.79 (3H, s), 3.55–3.59 (1H, m, H-3a), 3.42 (1H, td,
J = 11.5, 3.5 Hz, H-3b), 3.15 (1H, ddd,
J = 16.5, 11.5, 6.5 Hz, H-4a), 2.92 (1H, d,
J = 16.0 Hz, H-4b);
13C-NMR (CDCl
3)
δ: 155.7 (C-4'), 142.6 (C-1'), 134.4, 129.7, 129.5, 129.0, 127.1, 126.7, 121.0, 117.7 (C≡N), 114.8, 55.6 (C-1), 44.9 (C-3), 28.7 (C-4); positive ESI-MS
m/z: 238 [M−CN]
+, 265 [M+H]
+, 287 [M+Na]
+. The IR,
1H-NMR and
13C-NMR data are agreement with the literature data [
17].
2-Phenyl-1,2,3,4-tetrahydroisoquinoline-1-carbonitrile (
24): white solid; yield: 73%; m.p. 89–90 °C; IR (KBr)
υmax cm
−1: 2223 (s, C≡N), 1222, 1206 (C−N); UV (MeOH): λ
max (lg
ε) 245 (4.04) nm;
1H-NMR (CDCl
3)
δ: 7.39–7.43 (2H, m), 7.31–7.37 (3H, m), 7.27–7.30 (1H, m), 7.14 (1H, s), 7.13 (1H,s), 7.06 (1H, t,
J = 7.5 Hz), 5.56 (1H, s, H-1), 3.82 (1H, ddd,
J = 12.5, 6.0, 3.0 Hz, H-3a), 3.53 (1H, ddd,
J = 12.0, 10.5, 4.0 Hz, H-3b), 3.19 (1H, ddd,
J = 16.5, 10.5, 6.0 Hz, H-4a), 3.01 (1H, dt,
J = 16.0, 3.5 Hz, H-4b);
13C-NMR (CDCl
3)
δ: 148.4 (C-1'), 134.7, 129.7, 129.4, 128.8, 127.1, 126.9, 121.9, 117.8 (C≡N), 117.6, 53.2 (C-1), 44.2 (C-3), 28.6 (C-4); positive ESI-MS
m/z: 208 [M−CN]
+. The IR,
1H-NMR and
13C-NMR data are agreement with the literature data [
17].