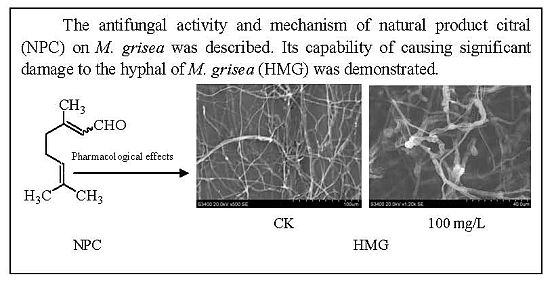
The Natural Product Citral Can Cause Significant Damage to the Hyphal Cell Walls of Magnaporthe grisea
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Citral on Fungal Hyphal Growth

2.2. Effects of Citral on Germination of Fungal Spores
| Citral Treatment(μg/mL) | Spore Germination Rate (%) | Germ Tube Length (μm) |
|---|---|---|
| 0 | 100.00 a | 175.67 a |
| 12.5 | 89.66 b | 123.78 b |
| 25 | 70.90 c | 91.11 c |
| 50 | 34.47 d | 54.44 d |
| 100 | 14.40 e | 30.11 e |
| 200 | 5.20 f | 12.67 f |
| 400 | 0.00 j | 0.00 j |
2.3. Electron Microscope Analysis of M. grisea


2.4. Effect of Citral on Chitinases Activity of M. grisea

3. Experimental Section
3.1. Chemicals and Samples
3.2. Impact of Citral on Fungal Hyphal Growth
3.3. Impact of Citral on Fungal Spore Germination
3.4. Morphological Study
3.5. Impact of Citral on Chitinases of M. grisea
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Skamnioti, P.; Gurr, S.J. Against the grain: Safeguarding rice from rice blast disease. Trends Biotechnol. 2009, 27, 141–150. [Google Scholar]
- Dean, R.A.; Talbot, N.J.; Ebbole, D.J.; Farman, M.L.; Mitchell, T.K.; Orbach, M.J.; Thon, M.; Kulkarni, R.; Xu, J.R.; Pan, H.; et al. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 2005, 434, 980–986. [Google Scholar]
- Motallebi, P.; Javan-Nikkhah, M.; Okhovvat, S.M. Characterization of Magnaporthe grisea populations associated with rice and weeds in Iran. Australas. Plant Path. 2013, 42, 693–700. [Google Scholar] [CrossRef]
- Tani, H.; Koshino, H.; Sakuno, E.; Nakajima, H. Botcinins A, B, C, and D, metabolites produced by Botrytis cinerea, and their antifungal activity against Magnaporthe grisea, a pathogen of rice blast disease. J. Nat. Prod. 2005, 68, 1768–1772. [Google Scholar] [CrossRef]
- Dean, R.; van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Pietro, A.D.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology . Mol. Plant Path. 2012, 13, 141–430. [Google Scholar]
- Zhang, Y.; Li, S.; Jiang, D.; Kong, L.; Zhang, P.; Xu, J. Antifungal activities of metabolites produced by a termite-associated Streptomyces canus BYB02. J. Agric. Food Chem. 2013, 61, 1521–1524. [Google Scholar]
- Zhang, C.Q.; Zhou, M.G. Isolation, characterization and preliminary genetic analysis of tricyclazole-resistant mutants of the rice blast fungus, Magnaporthe grise. J. Phytopathol. 2006, 154, 392–397. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Huang, X.; Wang, J.X.; Zhou, M.G. Resistance development in rice blast disease caused by Magnaporthe grisea to tricyclazole. Pestic. Biochem. Physiol. 2009, 94, 43–47. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Korsten, L. Biological control of postharvest diseases of fruits. Ann. Rev. Phytopathol. 2002, 40, 411–441. [Google Scholar] [CrossRef]
- Haas, D.; Keel, C. Regulation of antibiotic production in root-colonizing Pseudomonas spp. and relevance for biological control of plant disease. Ann. Rev. Phytopathol. 2003, 41, 117–153. [Google Scholar] [CrossRef]
- Teixeira, R.R.; Pereira, W.L.; Oliveira, A.F.C.; Silva, A.M.; Oliveira, A.S.; Silva, M.L.; Silva, C.C.; Paula, S.O. Natural products as source of potential dengue antivirals. Molecules 2014, 19, 8151–8176. [Google Scholar] [CrossRef]
- Yoon, M.Y.; Cha, B.; Kim, J.C. Recent trends in studies on botanical fungicides in agriculture. Plant Pathol. J. 2013, 29, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Z.T.; Li, R. Complexation and molecular microcapsules of Litsea cubeba essential oil with β-cyclodextrin and its derivatives. Eur. Food Res. Technol. 2009, 228, 865–873. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y. Chemical composition and antibacterial activity of essential oils from different parts of Litsea cubeba. Chem. Biodivers. 2010, 7, 229–235. [Google Scholar] [CrossRef]
- Liu, T.T.; Yang, T.S. Antimicrobial impact of the components of essential oil of Litsea cubeba from Taiwan and antimicrobial activity of the oil in food systems. Int. J. Food Microbiol. 2012, 156, 68–75. [Google Scholar] [CrossRef]
- Silva, C.B.; Guterres, S.S.; Weisheimer, V.; Schapoval, E.E.S. Antifungal activity of the Lemongrass oil and citral against Candida spp. Braz. J. Infect. Dis. 2008, 12, 63–66. [Google Scholar]
- Saddiq, A.A.; Khayyat, S.A. Chemical and antimicrobial studies of monoterpene: Citral. Pestic. Biochem. Physiol. 2010, 98, 89–93. [Google Scholar] [CrossRef]
- Aiemsaard, J.; Aiumlamai, S.; Aromdee, C.; Taweechaisupapongc, S.; Khunkitti, W. The effect of lemongrass oil and its major components on clinical isolate mastitis pathogens and their mechanisms of action on Staphylococcus aureus DMST 4745. Res. Vet. Sci. 2011, 91, 31–37. [Google Scholar] [CrossRef]
- Park, M.J.; Gwak, K.S.; Yang, I.; Kim, K.W.; Jeung, E.B.; Chang, J.W.; Choi, I.G. Effect of citral, eugenol, nerolidol and α-terpineol on the ultrastructural changes of Trichophyton mentagrophytes. Fitoterapia 2009, 80, 290–296. [Google Scholar] [CrossRef]
- Wuryatmo, E.; Klieber, A.; Scott, E.S. Inhibition of citrus postharvest pathogens by vapor of citral and related compounds in culture. J. Agric. Food Chem. 2003, 51, 2637–2640. [Google Scholar] [CrossRef]
- Ganjewala, D.; Guptam, A.K.; Muhury, R. An update on bioactive potential of a monoterpene aldehyde citral. JBAPN 2012, 2, 186–199. [Google Scholar]
- Daferera, D.J.; Ziogas, B.N.; Polissiou, M.G. The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp. and Clavibacter michiganensis subsp. Michiganensis. Crop Prot. 2003, 22, 39–44. [Google Scholar]
- Roger, A.A.; Thomas, R.H.; David, F.H.; Charles, T.M., Jr.; Randall, W.C.; Pierce, D.F. Structure-antifungal activity relationships among volatile C6 and C9 aliphatic aldehydes, ketones and alcohols. J. Agric. Food Chem. 1994, 4, 1563–1568. [Google Scholar]
- Witz, G. Biological interaction of α,β-unsaturated aldehydes. Free Radic. Biol. Med. 1989, 7, 333–349. [Google Scholar] [CrossRef]
- Maoz, M.; Neeman, I. Effect of Inulaviscosa extract on chitin systhesis in dermatophytes and Candida albicans. J. Ethnopharmacol. 2000, 71, 479–482. [Google Scholar] [CrossRef]
- Xiong, Z.Q.; Tu, X.R.; Wei, S.J.; Li, L.; Huang, X.H.; Lu, H. In vitro antifungal activity of antifungalmycin 702, a new polyene macrolide antibiotic, against the rice blast fungus Magnaporthe grise. Biotechnol. Lett. 2013, 35, 1475–1479. [Google Scholar]
- Arlorio, M.; Ludwig, A.; Boller, T.; Bonfante, P. Inhibition of fungal growth by plant chitinases and β-1,3-glucanases. Protoplasma 1992, 171, 34–43. [Google Scholar] [CrossRef]
- Gooday, G.W.; Zhu, W.Y.; O’Donnell, R.W. What are the roles of chitinases in the growing fungus. FEMS Microbiol. Lett. 1992, 100, 387–392. [Google Scholar] [CrossRef]
- Abbanat, D.; Leighton, M.; Maiese, W.; Jones, E.B.; Pearce, C.; Greenstein, M. Cell wall active antifungal compounds produced by the marine fungus Hypoxylon oceanicum LL-15G256. I. Taxonomy and fermentation. J. Antibiot. 1998, 51, 296–302. [Google Scholar]
- Adams, D.J. Fungal cell wall chitinases and glucanases. Microbiology 2004, 150, 2029–2035. [Google Scholar] [CrossRef]
- Mauch, F.; Mauch-Mani, B.; Boller, T. Antifungal hydrolases in pea tissue II. Inhibition of fungal growth by combinations of chitinase and β-1,3-glucanase. Plant Physiol. 1998, 88, 936–942. [Google Scholar]
- Sela-Buurlage, M.B.; Ponstein, A.S.; Bres-Vloemans, S.A.; Melchers, L.S.; van den Elzen, P.J.M.; Cornelissen, B.J.C. Only specific tobacco (nicotiana tabacum) chitinases and [β]-1,3-glucanases exhibit antifungal activity. Plant Physiol. 1993, 101, 857–863. [Google Scholar]
- Bruno, G.B.; Donzelli, G. Interaction of amonium, reducing sugar, and chitin regulates the expression of cell wall degrading enzymes in Trichoderma atroviride strain P1. Appl. Environ. Microbiol. 2001, 67, 5643–5647. [Google Scholar] [CrossRef]
- Zore, G.B.; Thakre, A.D.; Jadhav, S.; Karuppayil, S.M. Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine 2011, 18, 1181–1190. [Google Scholar] [CrossRef]
- Nitoda, T.; Kurumatani, H.; Kanzaki, H.; Kawazu, K. Improved bioassay method for Spodoptera litura chitinase inhibitors using a colloidal chitin powder with a uniform particle size as substrate. Pestic. Sci. 1999, 55, 563–565. [Google Scholar] [CrossRef]
- Bolar, J.P.; Norelli, J.L.; Harman, G.E.; Brown, S.K.; Aldwinckle, H.S. Synergistic activity of endochitinase and exochitinase from Trichoderma atroviride (T. harzianum) against the pathogenic fungus (Venturia inaequalis) in transgenic apple plants. Transgenic Res. 2001, 10, 533–543. [Google Scholar] [CrossRef]
- Reissig, J.L.; Strominger, J.L.; Leloir, L.F. A modified colorimetric method for the estimation of N-acetylamino sugars. J. Biol. Chem. 1955, 217, 956–966. [Google Scholar]
- Boller, T.; Gehri, A.; Mauch, F.; Vögeli, U. Chitinase in bean leaves: Induction by ethylene, purification, properties, and possible function. Planta 1983, 157, 22–31. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available (the citral was isolated from Litsea cubeba essential oils) from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.-Y.; Wu, X.-M.; Yin, X.-H.; Liang, J.-N.; Li, M. The Natural Product Citral Can Cause Significant Damage to the Hyphal Cell Walls of Magnaporthe grisea. Molecules 2014, 19, 10279-10290. https://doi.org/10.3390/molecules190710279
Li R-Y, Wu X-M, Yin X-H, Liang J-N, Li M. The Natural Product Citral Can Cause Significant Damage to the Hyphal Cell Walls of Magnaporthe grisea. Molecules. 2014; 19(7):10279-10290. https://doi.org/10.3390/molecules190710279
Chicago/Turabian StyleLi, Rong-Yu, Xiao-Mao Wu, Xian-Hui Yin, Jing-Nan Liang, and Ming Li. 2014. "The Natural Product Citral Can Cause Significant Damage to the Hyphal Cell Walls of Magnaporthe grisea" Molecules 19, no. 7: 10279-10290. https://doi.org/10.3390/molecules190710279