Two New Iridoids from Verbena officinalis L.
Abstract
:1. Introduction
2. Results and Discussion

| Position | 1 | 2 | ||
|---|---|---|---|---|
| 1H | 13C | 1H | 13C | |
| 1 | - | 176.2 | 5.94 (1H, d, J = 1.5 Hz) | 94.1 |
| 3 | 8.68 (1H, d, J = 0.5 Hz) | 163.5 | 7.37 (s) | 154.8 |
| 4 | - | 120.9 | - | 109.7 |
| 5 | 8.00 (1H, d. J = 0.5 Hz) | 154.7 | - | 71.7 |
| 6 | - | 175.9 | - | 198.4 |
| 7 | 2.71 (1H, dd, J = 6.5, 15.5 Hz)2.48 (1H, dd, J = 8.0, 15.5 Hz) | 40.0 | - | 149.3 |
| 8 | 3.26 (1H, m) | 29.6 | - | 137.2 |
| 9 | - | 135.8 | 3.20 (1H, like t, J = 1.5 Hz) | 53.7 |
| 10 | 1.25 (3H, d, J = 7.0 Hz) | 19.2 | 1.91 (3H, d, J = 1.5 Hz) | 11.9 |
| 11 | - | 165.0 | - | 167.8 |
| 1ꞌ | 4.45 (1H, d, J = 8.0 Hz) | 100.2 | ||
| 2ꞌ | 3.00–3.28 (1H, m) | 71.9 | ||
| 3ꞌ | 3.00–3.28 (1H, m) | 77.8 | ||
| 4ꞌ | 3.00–3.28 (1H, m) | 74.7 | ||
| 5ꞌ | 3.00–3.28 (1H, m) | 78.9 | ||
| 6ꞌ | 3.00–3.28 (1H, m)3.56 (1H, dd, J = 6.5, 12 Hz) | 63.1 | ||
| OCH3 | 3.83 (3H, s) | 52.9 | 3.61 (3H, s) | 52.1 |

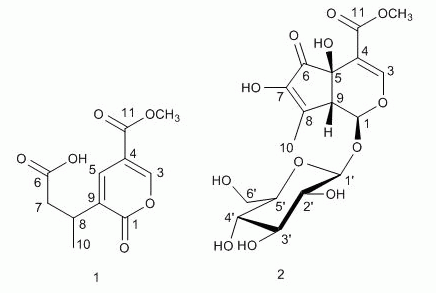
 = −94.5° (c = 0.012, MeOH), with a quasi-molecular ion peak at m/z 441.1001 [M+Na]+, indicating that the molecular formula of 2 was putatively C17H22O12, which was further confirmed by its 13C-NMR spectrum (Table 1). The UV absorption maximum at 238 nm suggested that 2 had a conjugated double bond and an enol ether, which are characteristic of the -OCO-C=CH-O- chromophore found in iridoids [15]. The 1H-NMR and 13C-NMR spectra of compound 2 showed similar features to those of hastatoside [12]. The differences between the 1H-NMR data of 2 and that of hastatoside were signals at δH 1.91 (3H, d, J = 1.5 Hz, H-10), 3.20 (1H, like t, J = 1.5 Hz, H-9), and the differences between the 13C-NMR data of 2 and that of hastatoside were signals at δC 137.2 (C-8), 149.3 (C-7), those suggested that 2 possesses a second conjugated double bond, and it can be assigned at C-7-C-8, it was confirmed by the change of chemical shifts of C-6 of compound 2 showed the remarkable upfield shifts by 18ppm, compared to that of hastatoside (δC 215), and the HMBC spectrum analysis (Figure 2) displayed the correlation peaks between H-10 and C-7. Furthermore, in HMBC spectrum, the olefinic proton at δH 7.37 (H-3) showed cross-peaks with C-11, C-5, and C-1, which suggested that the attachment hydroxyl group was assigned to C-5; A methine proton at δH 3.20 (1H, t, J = 1.5 Hz) correlated with C-4, 6, 7 and 10, which implied that the proton should be assigned at C-9; the anomeric proton at δH 4.45 (1H, d, J = 8.0 Hz) showed correlations with C-1, which suggested that the sugar moiety was located at C-1. The attachment of the hydroxyl group was assigned to C-7 due to the analysis of the molecular formula. In the meantime, it was proved by the 1H-NMR spectrum. In the 1H-NMR, there wasn’t another olefinic proton except for H-3. Confirmation of the stereochemistry of its stereogenic centers was achieved by analysis of J values and comparison with 13C-NMR literature data, especially those for chiral centers at C-1, C-5, and C-9, which indicated the β configuration of the hydroxyl group at C-5, consistent with the configuration of this substituent in hastatoside, The cis junction between the two rings and the O-glycosyl residue at C-1 is biosynthetically supported with a β-configuration [16]. From the above information, compound 2 was thus identified as 7-hydroxydehydrohastatoside.
= −94.5° (c = 0.012, MeOH), with a quasi-molecular ion peak at m/z 441.1001 [M+Na]+, indicating that the molecular formula of 2 was putatively C17H22O12, which was further confirmed by its 13C-NMR spectrum (Table 1). The UV absorption maximum at 238 nm suggested that 2 had a conjugated double bond and an enol ether, which are characteristic of the -OCO-C=CH-O- chromophore found in iridoids [15]. The 1H-NMR and 13C-NMR spectra of compound 2 showed similar features to those of hastatoside [12]. The differences between the 1H-NMR data of 2 and that of hastatoside were signals at δH 1.91 (3H, d, J = 1.5 Hz, H-10), 3.20 (1H, like t, J = 1.5 Hz, H-9), and the differences between the 13C-NMR data of 2 and that of hastatoside were signals at δC 137.2 (C-8), 149.3 (C-7), those suggested that 2 possesses a second conjugated double bond, and it can be assigned at C-7-C-8, it was confirmed by the change of chemical shifts of C-6 of compound 2 showed the remarkable upfield shifts by 18ppm, compared to that of hastatoside (δC 215), and the HMBC spectrum analysis (Figure 2) displayed the correlation peaks between H-10 and C-7. Furthermore, in HMBC spectrum, the olefinic proton at δH 7.37 (H-3) showed cross-peaks with C-11, C-5, and C-1, which suggested that the attachment hydroxyl group was assigned to C-5; A methine proton at δH 3.20 (1H, t, J = 1.5 Hz) correlated with C-4, 6, 7 and 10, which implied that the proton should be assigned at C-9; the anomeric proton at δH 4.45 (1H, d, J = 8.0 Hz) showed correlations with C-1, which suggested that the sugar moiety was located at C-1. The attachment of the hydroxyl group was assigned to C-7 due to the analysis of the molecular formula. In the meantime, it was proved by the 1H-NMR spectrum. In the 1H-NMR, there wasn’t another olefinic proton except for H-3. Confirmation of the stereochemistry of its stereogenic centers was achieved by analysis of J values and comparison with 13C-NMR literature data, especially those for chiral centers at C-1, C-5, and C-9, which indicated the β configuration of the hydroxyl group at C-5, consistent with the configuration of this substituent in hastatoside, The cis junction between the two rings and the O-glycosyl residue at C-1 is biosynthetically supported with a β-configuration [16]. From the above information, compound 2 was thus identified as 7-hydroxydehydrohastatoside.3. Experimental
3.1. Plant Materials
3.2. General Procedure and Regents
3.3. Extraction and Isolation
3.4. Spectra Data
 = −57.2° (c = 0.062, MeOH). IR (KBr, cm−1): 3328, 1736, 1720, 1685, 1648, 1624, 1562, 1540, 1458, 1440, 1293, 1133. ESI-MS m/z 241.27[M+H]+, 279.22[M+Na]+, 502.93[2M+Na]+. HRESI-MS m/z 263.0530 [M+Na]+ (C11H12O6Na). 1H-NMR and 13C-NMR data see Table 1.
= −57.2° (c = 0.062, MeOH). IR (KBr, cm−1): 3328, 1736, 1720, 1685, 1648, 1624, 1562, 1540, 1458, 1440, 1293, 1133. ESI-MS m/z 241.27[M+H]+, 279.22[M+Na]+, 502.93[2M+Na]+. HRESI-MS m/z 263.0530 [M+Na]+ (C11H12O6Na). 1H-NMR and 13C-NMR data see Table 1. = −94.5° (c = 0.012, MeOH). IR (KBr, cm−1): 3318, 1733, 1722, 1652, 1628, 1560, 1535, 1448, 1288, 1074. ESI-MS m/z 417 [M−H]−, HRESI-MS m/z 441.1001 [M+Na]+ (C17H22O12Na). 1H-NMR and 13C-NMR data see Table 1.
= −94.5° (c = 0.012, MeOH). IR (KBr, cm−1): 3318, 1733, 1722, 1652, 1628, 1560, 1535, 1448, 1288, 1074. ESI-MS m/z 417 [M−H]−, HRESI-MS m/z 441.1001 [M+Na]+ (C17H22O12Na). 1H-NMR and 13C-NMR data see Table 1.4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Editor Committee of National Chinese Medical Manage Bureau. Chinese Herb; Shanghai Science and Technology Publisher: Shanghai, China, 1999; Volume 7, pp. 950–951. [Google Scholar]
- Reynaud, J.; Couble, A.; Raynaud, J. O-glycosylflavonoids of Verbena officinalis L. Pharm. Acta Helv. 1992, 67, 216–217. [Google Scholar]
- Deepak, M.; Handa, S.S. 3α,24-dihydroxy-urs-12-en-28-oic acid from Verbena officinalis. Phytochemistry 1998, 49, 269–271. [Google Scholar] [CrossRef]
- Xin, F.; Jin, Y.S.; Sha, Q.; Xu, W.; Zu, Z.; Li, Y.S. Chemical constituents isolated from Verbena officinalis. Mod. Chin.Med. 2008, 10, 21–23. [Google Scholar]
- Damtoft, S.; Jensen, S.R.; Nielsen, B.J. Iridoids in Verbena. Taxon 1979, 28, 525–528. [Google Scholar] [CrossRef]
- Jensen, S.R.; Kirk, J.O.; Nielsen, B.J. Biosynthesis of iridoid glucosicde cornin in Verbena officinalis. Phytochemistry 1989, 28, 97–105. [Google Scholar] [CrossRef]
- Makino, Y.; Kondo, S.; Nishimuna, Y.; Tsukamoto, Y.; Huang, Z.L.; Urade, Y. Hastatoside and verbenalin are sleep-promoting components in Verbena officinalis. Sleep Biol. Rhythms 2009, 7, 211–217. [Google Scholar] [CrossRef]
- Bilia, A.R.; Giomi, M.; Innocenti, M.; Gallori, S.; Vincieri, F.F. HPLC-DAD-ESI-MS analysis of the constituents of aqueous preparations of verbena and lemon verbena and evaluation of the antioxidant activity. J. Pharm. Biomed. 2008, 46, 463–470. [Google Scholar] [CrossRef]
- Singh, B.; Saxena, A.K.; Chandan, B.K.; Anand, K.K.; Suri, O.P.; Suri, K.A.; Satti, N.K. Hepatoprotective activity of verbenalin on experimental liver damage in rodents. Fitoterapia 1998, 69, 135–140. [Google Scholar]
- Shu, J.C.; Chou, G.X.; Wang, Z.T. Quality control of Herba Verbenae. Chin. J. Mod. Appl. Pharm. 2011, 28, 433–436. [Google Scholar]
- Teborg, D.; Junior, P. Iridoid glucosides from Pestemon nitidus. Planta Med. 1991, 57, 184–185. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, Y.M.; Luan, X.H. Studies on the chemical constituents in herb of Verbena officinalis. China J. Chin.Mater. Med. 2005, 30, 268–269. [Google Scholar]
- Zhang, T.; Luan, J.L.; Lv, Z.M. Studies on iridoid glucosides from Verbena officinalis. Chin. Tradit. Herbal Drug. 2000, 31, 721–723. [Google Scholar]
- Rimpler, H.; Schäfer, B.Z. Hastatoside, a new iridoid from Verbena hastata L. and Verbena officinalis L. Z. Naturforsch. 1979, 34C, 311–318. [Google Scholar]
- Dinda, B.; Debnath, S.; Harigaya, Y. Naturally occurring Iridoids. A review. Chem. Pharm. Bull. 2007, 55, 159–222. [Google Scholar] [CrossRef]
- Bianco, A. The chemistry of iridoids. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier Sciences Publishers: Amsterdam, The Netherlands, 1990; Volume 7, p. 454. [Google Scholar]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, J.; Chou, G.; Wang, Z. Two New Iridoids from Verbena officinalis L. Molecules 2014, 19, 10473-10479. https://doi.org/10.3390/molecules190710473
Shu J, Chou G, Wang Z. Two New Iridoids from Verbena officinalis L. Molecules. 2014; 19(7):10473-10479. https://doi.org/10.3390/molecules190710473
Chicago/Turabian StyleShu, Jicheng, Guixin Chou, and Zhengtao Wang. 2014. "Two New Iridoids from Verbena officinalis L." Molecules 19, no. 7: 10473-10479. https://doi.org/10.3390/molecules190710473