Antioxidant Activity and Acetylcholinesterase Inhibition of Grape Skin Anthocyanin (GSA)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Radical Scavenging Activity
| Extract | DPPH Radical | ABTS Radical | Metal Chelating | |||
|---|---|---|---|---|---|---|
| Scavenging Activity (%) | IC50 Value µ g/mL | Scavenging Activity (%) | IC50 Value µ g/mL | Scavenging Activity (%) | IC50 Value µ g/mL | |
| GSA | 95.54 ± 0.43 a | 95.14 ± 1.13 a | 97.67 ± 1.009 a | 62.74 ± 0.43 a | 56.26 ± 1.67 a | 180.49 ± 19.40 a |
| Positive control | 97.75 ± 0.28 b (Vitamin C) | 71.50 ± 1.05 b | 99.78 ± 0.34 b (Vitamin C) | 20.32 ± 0.20 b | 89.82 ± 2.69 b (EDTA 100 µM) | 7.089 ± 0.78 b |
2.2. Fe2+ Chelation
2.3. Oxidative Hemolysis Inhibition Assay

2.4. Oxidative DNA Damage Prevention

2.5. Evaluation of Antioxidation of GSA Using a Mitochondria-Based Assay

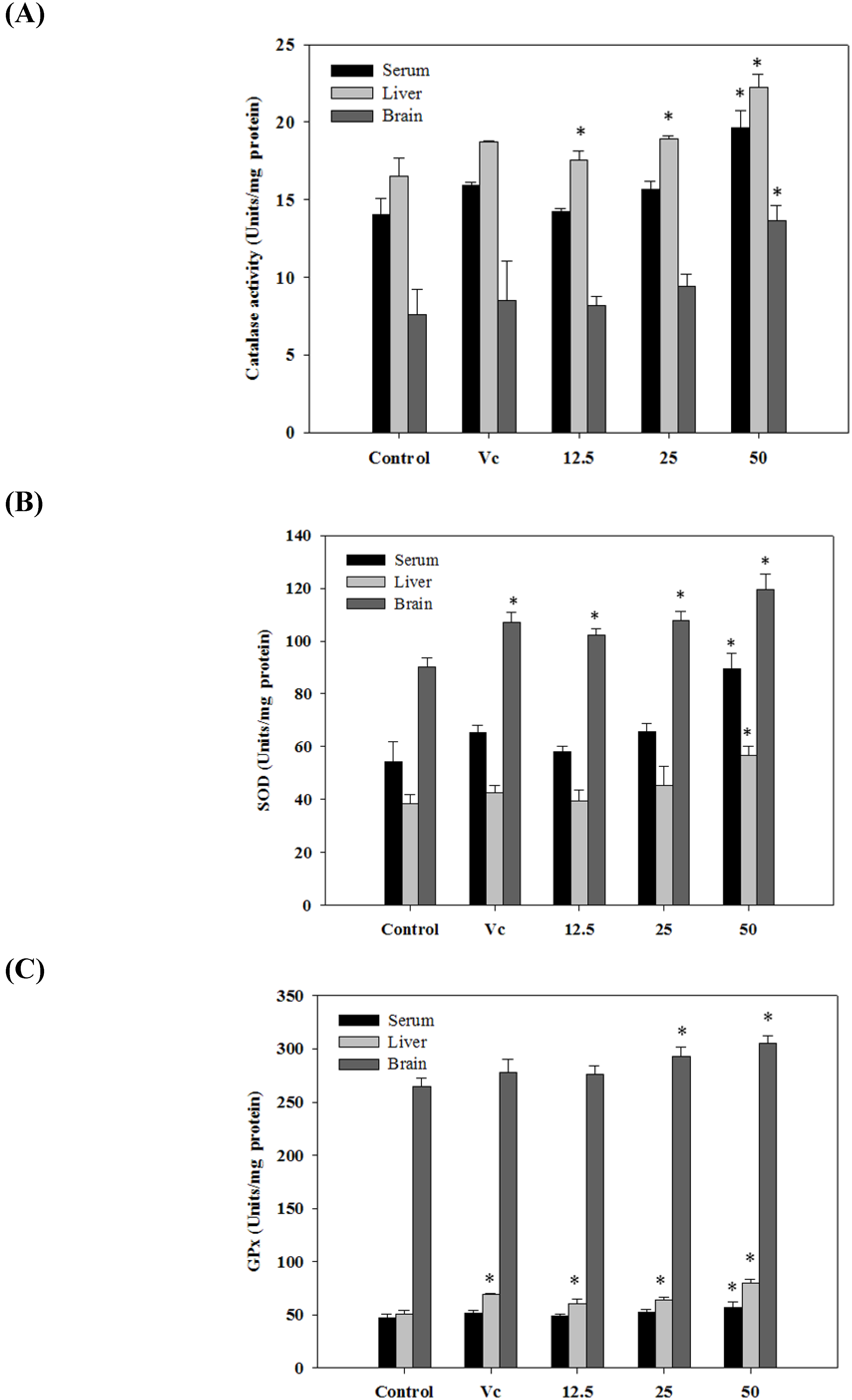
2.6. Antioxidant Activities in Vivo
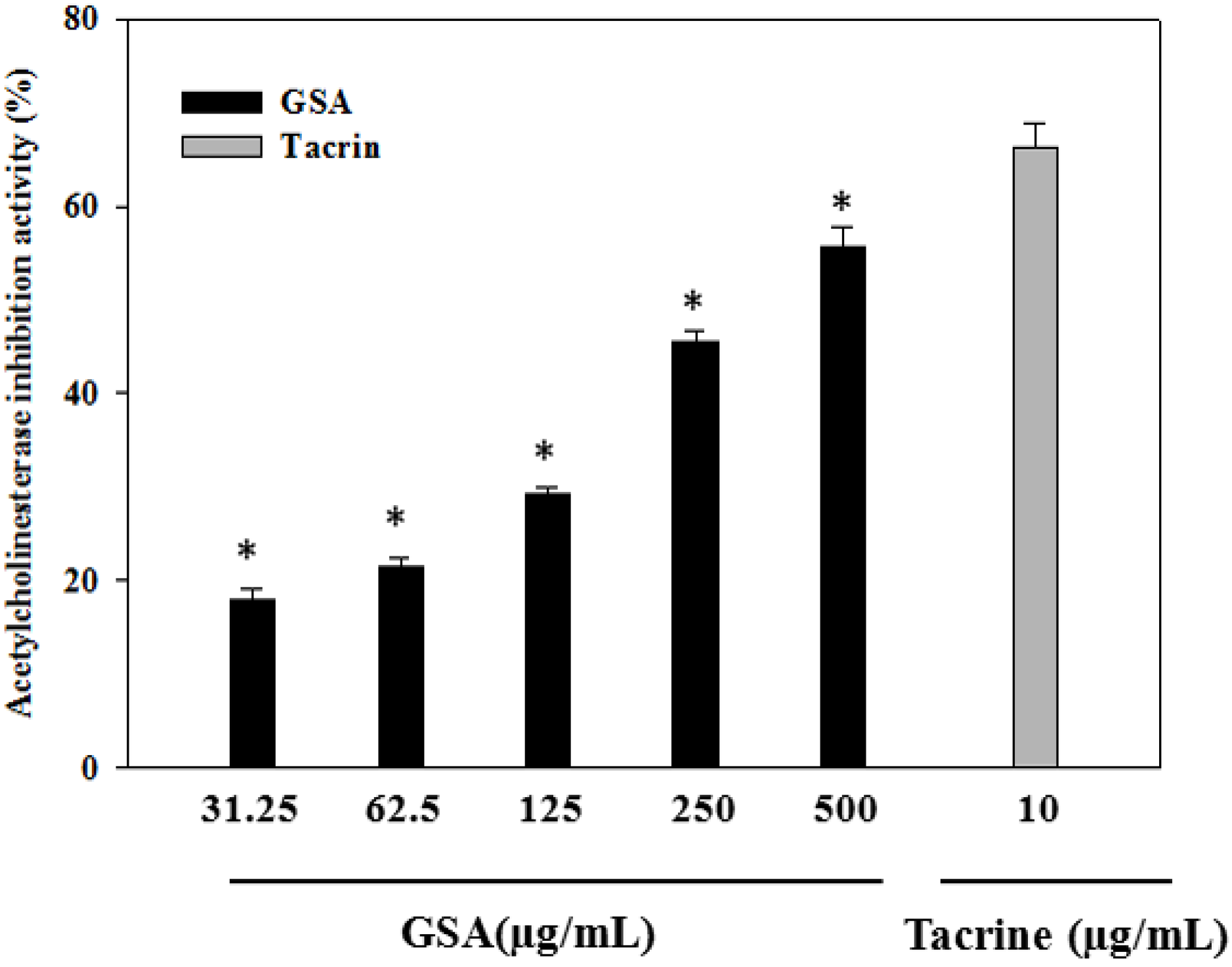
2.7. In Vitro Cholinesterase Inhibition
3. Experimental Section
3.1. Samples and Chemicals
3.2. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activities
3.3. 2,2'-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) Radical-Scavenging Activity
3.4. Fe2+ Chelation Assay
3.5. Oxidative Hemolysis Inhibition Assay
3.6. Assay for Effects of GSA on DNA oxidative damage
3.7. Mitochondria-Based Assay
3.7.1. Isolation of Mitochondria from Liver
3.7.2. Mitochondrial Reactive Oxygen Species (ROS) Measurements
3.8. In Vivo Antioxidant Activity
3.8.1. Animals and Experimental Design
3.8.2. Biochemical Assay
3.9. In Vitro Anticholinesterase Inhibition Assay
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cannizzo, E.S.; Clement, C.C.; Morozova, K.; Valdor, R.; Kaushik, S.; Almeida, L.N.; Follo, C.; Sahu, R.; Cuervo, A.M.; Macian, F.; et al. Age-related oxidative stress compromises endosomal proteostasis. Cell Rep. 2012, 2, 136–149. [Google Scholar] [CrossRef]
- Matsuda, T.; Kanki, T.; Tanimura, T.; Kang, D.; Matsuura, E.T. Effects of overexpression of mitochondrial transcription factor A on lifespan and oxidative stress response in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2013, 430, 717–721. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp Physiol. 1997, 82, 291–295. [Google Scholar]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Gabrovska, K.; Marinov, I.; Godjevargova, T.; Portaccio, M.; Lepore, M.; Grano, V.; Dianoc, N; Mita, G.M. The influence of the support nature on the kinetics parameters, inhibition constants and reactivation of immobilized acetylcholinesterase. Int. J. Biol. Macromol. 2008, 43, 339–345. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, S.; Valentao, P.; Andrade, P.B.; Romano, A. Accumulation of phenolic compounds in in vitro cultures and wild plants of Lavandula viridis L'Hér and their antioxidant and anti-cholinesterase potential. Food Chem. Toxicol. 2013, 57, 69–74. [Google Scholar] [CrossRef]
- Tang, Y.; Gao, C.; Xing, M.; Li, Y.; Zhu, L.; Wang, D.; Yang, X.; Liu, L.; Yao, P. Quercetin prevents ethanol-induced dyslipidemia and mitochondrial oxidative damage. Food Chem. Toxicol. 2012, 50, 1194–1200. [Google Scholar] [CrossRef]
- Suhaj, M. Spice antioxidants isolation and their antiradical activity: A review. J. Food Compos. Anal. 2006, 19, 531–537. [Google Scholar] [CrossRef]
- Liazid, A.; Guerrero, R.F.; Cantos, E.; Palma, M.; Barroso, C.G. Microwave assisted extraction of anthocyanins from grape skins. Food Chem. 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Flamini, R.; Mattivi, F.; Rosso, M.D; Arapitsas, P.; Bavaresco, L. Advanced knowledge of three important classes of grape phenolics: Anthocyanins, stilbenes and flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Saucier, C.; Glories, Y. Grape and wine phenolics: History and perspective. Am. J. Enol. Vitic. 2006, 57, 239–248. [Google Scholar]
- Choi, J.Y.; Lee, S.J.; Lee, S.J.; Park, S.; Lee, J.H.; Shim, J.H.; Abd El-Aty, A.M.; Jin, J.S.; Jeong, E.D.; Lee, W.S.; et al. Analysis and tentative structure elucidation of new anthocyanins in fruit peel of Vitis coignetiae Pulliat (meoru) using LC-MS/MS: Contribution to the overall antioxidant Activity . J. Sep. Sci. 2010, 33, 1192–1197. [Google Scholar]
- Manfra, M.; de Nisco, M.; Bolognese, A.; Nuzzo, V.; Sofo, A.; Scopa, A.; Santi, L.; Tenore, G.C.; Novellino, E. Anthocyanin composition and extractability in berry skin and wine of Vitis vinifera L. cv. Aglianico. J. Sci. Food Agric. 2011, 91, 2749–2755. [Google Scholar] [CrossRef]
- Cai, H.; Marczylo, T.H.; Teller, N.; Brown, K.; Steward, W.P.; Marko, D.; Gescher, A.J. Anthocyanin-rich red grape extract impedes adenoma development in the ApcMin mouse: Pharmacodynamic changes and anthocyanin levels in the murine biophase. Eur. J. Cancer 2010, 46, 811–817. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ichiyanagi, T.; Komiyama, T.; Sato, S.; Konishi, T. Effects of anthocyanins on psychological stress-induced oxidative stress and neurotransmitter status. J. Agric. Food Chem. 2008, 56, 7545–7550. [Google Scholar] [CrossRef]
- Gutierres, J.M; Carvalho, F.B.; Schetinger, M.R.; Marisco, P.; Agostinho, P.; Rodrigues, M.; Rubin, M.A.; Schmatz, R.; da Silva, C.R.; de P Cognato, G.; et al. Anthocyanins restore behavioral and biochemical changes caused by streptozotocin-induced sporadic dementia of Alzheimer’s type. Life Sci. 2013, 96, 7–17. [Google Scholar]
- Shukitt-Hale, B.; Cheng, V.; Joseph, J.A. Effects of blackberries on motor and cognitive function in aged rats. Nutr. Neurosci. 2009, 12, 135–40. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.D.S.S.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian wine making. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, Y.; Gao, H.; Yuan, J.; Feng, F.; Cao, W.; Zheng, J. Protective effect of extract of Crataegus pinnatifida pollen on DNA damage response to oxidative stress. Food Chem. Toxicol. 2013, 59, 709–714. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Oxygen toxicology, oxygen radicals, transition metals and disease. J. Biochem. 1984, 219, 1–4. [Google Scholar]
- Takebayashi, J.; Chen, J.; Tai, A. A method for evaluation of antioxidant activity based on inhibition of free radical-induced erythrocyte hemolysis. Methods Mol. Biol. 2010, 594, 287–296. [Google Scholar] [CrossRef]
- Carvalho, M.; Ferreira, P.J.; Mendes, V.S.; Silva, R.; Pereira, J.A.; Jeronimo, C.; Silva, B.M. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem. Toxicol. 2010, 48, 441–447. [Google Scholar] [CrossRef]
- Mendes, L.; Freitas, V.; Baptista, P.; Carvalho, M. Comparative antihemolytic and radical scavenging activities of strawberry tree (Arbutus unedo L.) leaf and fruit. Food Chem. Toxicol. 2011, 49, 2285–2291. [Google Scholar] [CrossRef]
- Devasagayam, T.P.A.; Subramanian, M.; Singh, B.B.; Ramanathan, R.; Das, N.P. Protection of plasmid pBR322 DNA by flavonoids against single strand breaks induced by singlet molecular oxygen. J. Photochem. Photobiol. B: Biol. 1995, 30, 97–103. [Google Scholar] [CrossRef]
- Noroozi, M.; Angerson, W.J.; Lean, M.E.J. Effects of flavonoids and vitamin C on oxidative damage to human lymphocytes. Am. J. Clin. Nutr. 1998, 67, 1210–1218. [Google Scholar]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Brown, K.; Xie, S.; Qiu, X.; Mohrin, M.; Shin, J.; Liu, Y.; Zhang, D.; Scadden, D.T.; Chen, D. SIRT3 reverses aging-associated degeneration. Cell Rep. 2013, 3, 319–327. [Google Scholar] [CrossRef]
- Ushio-Fukai, M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid. Redox Signal. 2009, 11, 1289–1299. [Google Scholar]
- Bass, D.; Parce, J.W.; Dechatelet, L.R.; Szejda, P.; Seeds, M.; Thomas, M. Flow cytometric studies of oxidative product formation by neutrophils: A graded response to membrane stimulation. J. Immunol. 1983, 130, 1910–1917. [Google Scholar]
- Liu, J.; Jia, L.; Kan, J.; Jin, C.H. In vitro and in vivo antioxidant activity of ethanolic extract of white button mushroom (Agaricus bisporus). Food Chem. Toxicol. 2013, 51, 310–316. [Google Scholar] [CrossRef]
- Hwang, J.W.; Kim, E.K.; Lee, S.J.; Kim, Y.S.; Moon, S.H.; Jeon, B.T.; Sung, S.H.; Kim, E.T.; Park, P.J. Antioxidant activity and protective effect of anthocyanin oligomers on H2O2-triggered G2/M arrest in retinal cells. J. Agric. Food Chem. 2012, 60, 4282–4288. [Google Scholar] [CrossRef]
- Puiggros, F.; Sala, E.; Vaque, M.; Ardevol, A.; Blay, M.; Fernández-Larrea, J.; Arola, L.; Bladé, C.; Pujadas, G.; Salvadó, M.J. In vivo, in vitro, and in silico studies of CU/ZN-superoxide dismutase regulation by molecules in grape seed procyanidin extract. J. Agric. Food Chem. 2009, 57, 3934–3942. [Google Scholar] [CrossRef]
- Faria, A.; Pestana, D.; Monteiro, R.; Teixeira, D.; Azevedo, J.; Freitas, V.D. Bioavailability of Anthocyanin-pyruvic Acid Adducts in Rat. In Proceedings of the International Conference on Polyphenols and Health, Yorkshire, Leeds, UK, 7–10 December 2009; pp. 170–171.
- Faria, A.; Pestana, D.; Azevedo, J.; Martel, F.; de Freitas, V.; Azevedo, I.; Mateus, N.; Calhau, C. Absorption of anthocyanins through intestinal epithelial cells - Putative involvement of GLUT2. Mo.l Nutr. Food Res. 2009, 53, 1430–1437. [Google Scholar] [CrossRef]
- Passamonti, S.; Vrhovsek, U.; Vanzo, A.; Mattivi, F. Fast access of some grape pigments to the brain. J. Agric. Food Chem. 2005, 53, 7029–7034. [Google Scholar] [CrossRef]
- Talave'ra, S.; Felgines, C.; Texier, O.; Besson, C.; Gil-Izquierdo, A.; Lamaison, J.L.; Rémésy, C. Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain. J. Agric. Food Chem. 2005, 53, 3902–3908. [Google Scholar] [CrossRef]
- Bitsch, R.; Netzel, M.; Frank, T.; Strass, G.; Bitsch, I. Bioavailability and biokinetics of anthocyanins from red grape juice and red wine. J. Biomed. Biotechnol. 2004, 5, 293–298. [Google Scholar]
- Tabet, N. Acetylcholinesterase inhibitors for Alzheimer’s disease: Antiinflammatories in acetylcholine clothing. Age Ageing 2006, 35, 336–338. [Google Scholar] [CrossRef]
- Costa, P.; Grosso, C.; Gonçalves, S.; Andrade, P.B.; Valentão, P.; Bernardo-Gil, M.G.; Romano, A. Supercritical fluid extraction and hydrodistillation for the recovery of bioactive compounds from Lavandula viridis L’Hér. Food Chem. 2012, 135, 112–121. [Google Scholar] [CrossRef]
- Szwajgier, D. Anticholinesterase activities of selected polyphenols—a short report. Pol. J. Food Nutr. Sci. 2014, 64, 59–64. [Google Scholar]
- Ryu, H.W.; Curtis-Long, M.J.; Jung, S.; Jeong, I.Y.; Kim, D.S.; Kang, K.Y.; Park, K.H. Anticholinesterase potential of flavonols from paper mulberry (Broussonetia papyrifera) and their kinetic studies. Food Chem. 2012, 132, 1244–1250. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, R. A survey of plants for presence of cholinesterase activity. Phytochemistry 1997, 46, 827–831. [Google Scholar] [CrossRef]
- Bekir, J.; Mars, M.; Souchard, J.P.; Bouajila, J. Assessment of antioxidant, anti-inflammatory, anti-cholinesterase and cytotoxic activities of pomegranate (Punica granatum) leaves. Food Chem. Toxicol. 2013, 55, 470–475. [Google Scholar] [CrossRef]
- Gutierres, J.M.; Carvalho, F.B.; Schetinger, M.R.; Agostinho, P.; Marisco, P.C.; Vieira, J.M.; Rosa, M.M.; Bohnert, C.; Rubin, M.A.; Morsch, V.M.; et al. Neuroprotective effect of anthocyanins on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia in rats. Int. J. Dev. Neurosci. 2014, 33, 88–97. [Google Scholar] [CrossRef]
- Fournand, D.; Vicens, A.; Sidhoum, L.; Souquet, J.M.; Moutounet, M.; Cheynier, V. Accumulation and extractability of grape skin tannins and anthocyanins at different advanced physiological stages. J. Agric. Food Chem. 2006, 54, 7331–7338. [Google Scholar] [CrossRef]
- He, K.; Li, X.; Ye, X.; Yuan, L.; Li, X.; Chen, X.; Deng, Y. A mitochondria-based method for the determination of antioxidant activities using 2',7'-dichlorofluorescin diacetate oxidation. Food Res. Int. 2012, 48, 454–461. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courteney, K.D.; Valentino, A.J.; Featherstone, R.M. A new and rapid colorimeteric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pervin, M.; Hasnat, M.A.; Lee, Y.M.; Kim, D.H.; Jo, J.E.; Lim, B.O. Antioxidant Activity and Acetylcholinesterase Inhibition of Grape Skin Anthocyanin (GSA). Molecules 2014, 19, 9403-9418. https://doi.org/10.3390/molecules19079403
Pervin M, Hasnat MA, Lee YM, Kim DH, Jo JE, Lim BO. Antioxidant Activity and Acetylcholinesterase Inhibition of Grape Skin Anthocyanin (GSA). Molecules. 2014; 19(7):9403-9418. https://doi.org/10.3390/molecules19079403
Chicago/Turabian StylePervin, Mehnaz, Md. Abul Hasnat, Yoon Mi Lee, Da Hye Kim, Jeong Eun Jo, and Beong Ou Lim. 2014. "Antioxidant Activity and Acetylcholinesterase Inhibition of Grape Skin Anthocyanin (GSA)" Molecules 19, no. 7: 9403-9418. https://doi.org/10.3390/molecules19079403





