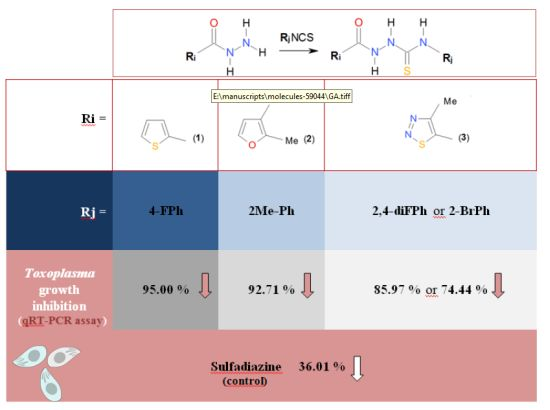
1,4-Disubstituted Thiosemicarbazide Derivatives are Potent Inhibitors of Toxoplasma gondii Proliferation
Abstract
:1. Introduction

2. Results and Discussion
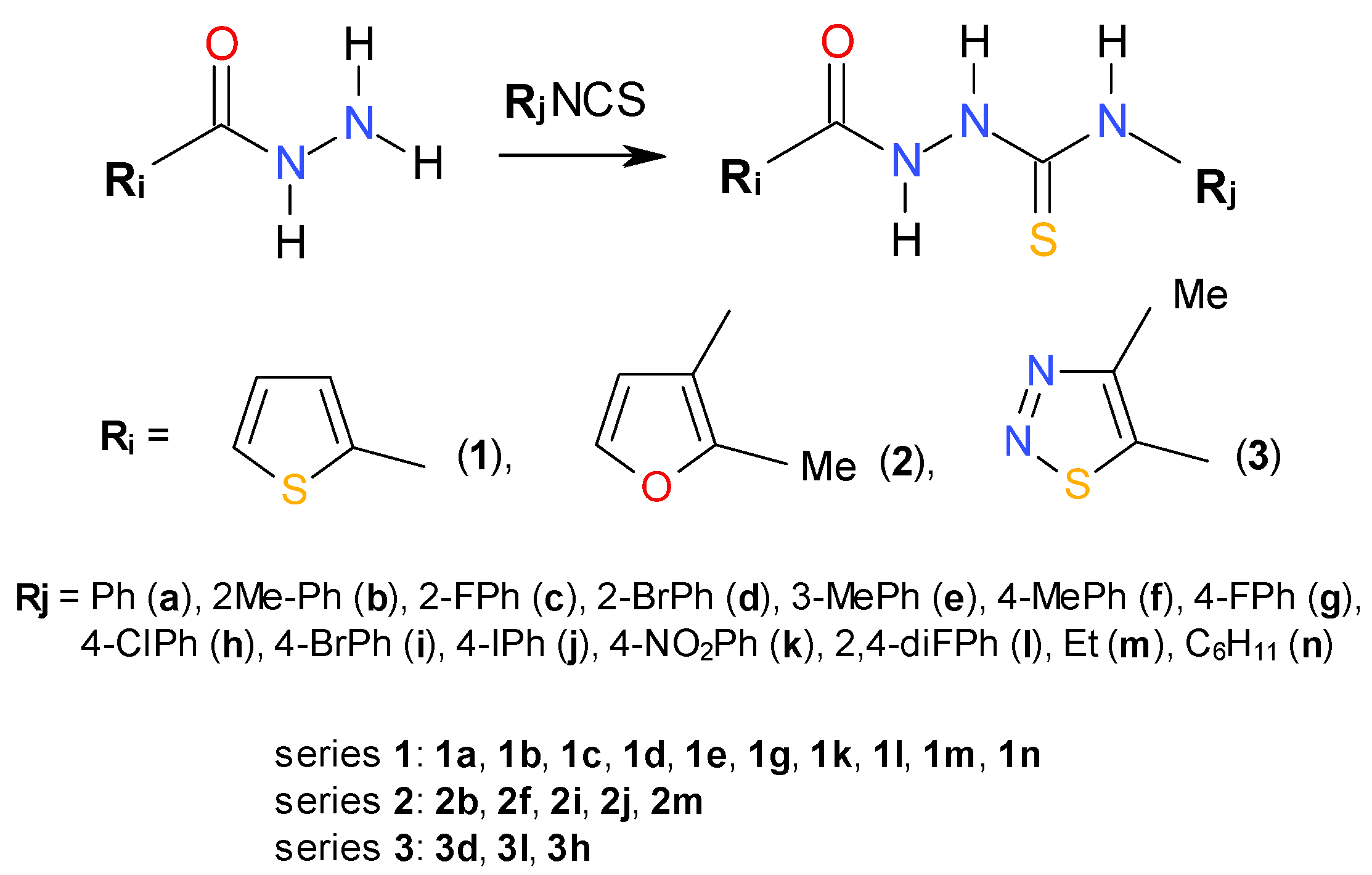
2.1. Chemistry

2.2. In Vitro Anti-Toxoplasma gondii Activity of the Title Compounds
| Cmpd. No. | Assay: A. [3H] Uracil Incorporation B. qRT-PCR | Concentration [µg/mL] | IC50 [µg/mL] | ||||
|---|---|---|---|---|---|---|---|
| 100 | 50 | 10 | 5 | 1 | |||
| 1g | A. | nt | 12.62 ± 4.78 * | 44.71 ± 16.60 * | 74.94 ± 7.00 * | 96.14 ± 14.54 | 33.17 |
| B. | nt | 5.00 ± 2.56 * | 61.01 ± 11.23 * | nt | nt | nt | |
| 2b | A. | 8.10 ± 2.78 * | 58.78 ± 11.49 * | 112.25 ± 22.91 * | 107.95 ± 24.88 | 98.24 ± 23.83 | 59.00 |
| B. | 7.29 ± 1.56 * | 41.37 ± 9.98 * | nt | nt | nt | nt | |
| 3d | A. | 36.52 ± 5.16 * | 63.82 ± 5.32 * | 83.84 ± 19.01 | 84.64 ± 15.68 | 90.03 ± 14.61 | 74.93 |
| B. | 25.56 ± 6.32 * | 75.13 ± 10.10 * | nt | nt | nt | nt | |
| 3l | A. | 37.20 ± 7.47 * | 88.34 ± 14.40 * | 103.21 ± 19.27 | 85.76 ± 19.71 | 84.59 ± 16.61 | 92.28 |
| B. | 14.08 ± 3.35 * | 89.32 ± 15.74 * | nt | nt | nt | nt | |
| sulfadiazine | A. | 71.53 ± 8,94 * | 78.73 ± 8,29 * | 82.14 ± 11,26 | 79.18 ± 6,29 * | 90.37 ± 11,65 * | >500 ** |
| B. | 63.99 ± 10.58 * | nt | nt | nt | nt | nt | |
| 2m | A. | 57.59 ± 15.02 * | 66.49 ± 11.38 * | 60.61 ± 9.56 * | 75.74 ± 14.62 | 98.00 ± 19.53 | 191.04 |
| B. | 53.03 ± 9.87 * | 51.03 ± 7.02 * | nt | nt | nt | nt | |

2.3. Cytotoxicity of 1g, 2b, 2m, 3d, and 3l against L929 Cells
| Cmpd. No. | Concentration [µg/mL] | CC30 [µg/mL] | |||||
|---|---|---|---|---|---|---|---|
| 500 | 100 | 50 | 10 | 5 | 1 | ||
| 1g | 39.76 ± 5.83 * | 67.00 ± 11.38 * | 79.05 ± 2.89 * | 97.32 ± 4.98 | 99.51 ± 5.16 | 102.52 ± 2.69 | 210.04 |
| 2b | 30.28 ± 0.86 | 78.22 ± 1.61 * | 84.54 ± 1.43 * | 91.93 ± 5.21 * | 94.01 ± 1.78 * | 95.27 ± 9.52 | 187.51 |
| 3d | 41.45 ± 5.16 * | 79.53 ± 5.82 * | 90.88 ± 6.71 * | 97.51 ± 5.98 | 99.14 ± 0.82 | 102.36 ± 3.89 | 242.56 |
| 3l | 48.02 ± 10.12 * | 89.55 ± 3.44 * | 99.04 ± 10.02 * | 95.89 ± 0.39 * | 92.87 ± 0.23 * | 96.54 ± 0.69 | 285.52 |
| 2m | 80.40 ± 2.74 * | 92.17 ± 2.31 * | 90.49 ± 2.99 * | 94.05 ± 5.13 | 94.44 ± 2.62 | 104.05 ± 0.12 | >500 |
2.4. Antibacterial Activity and Inhibitory Potency against Bacterial Type IIA Topoisomerases

3. Experimental
3.1. General Information
3.2. Chemistry: General Procedure for Synthesis of 1-Substituted-4-arylthiosemicarbazides
3.3. Assay in Vitro for Anti-T. gondii Activity
3.3.1. Animals
3.3.2. Parasites
3.3.3. Influence of Thiosemicarbazide Derivatives on T. gondii Proliferation
3.4. Quantitative Real-Time PCR
3.4.1. DNA Extraction
3.4.2. Detection in Infected Cells in the Presence of Tested Compounds
3.5. Cytotoxic Assay
3.5.1. Cell Culture
3.5.2. Preparation of Compounds
3.5.3. Cell Viability Assay
3.6. Antibacterial Assay
3.7. Inhibition of Bacterial Type IIA Topoisomerases
3.7.1. Supercoiling Assays
3.7.2. Decatenation Assays
3.8. Data Analysis
3.9. Computational Details
3.10. Docking Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Petersen, E.; Dubey, J.P. Biology of Toxoplasma gondii. In Toxoplasmosis: A Comprehensive Clinical Guide; Joynson, D.H.M., Wreghitt, T., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 1–49. [Google Scholar]
- Robert-Gangneux, F.; Dardé, M.L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar]
- Contini, C. Clinical and diagnostic management of toxoplasmosis in the immunocompromised patient. Parassitologia 2008, 50, 45–50. [Google Scholar]
- Ambroise-Thomas, P.; Pelloux, H. Toxoplasmosis-congenital and in immunocompromised patients: A parallel. Parasitol. Today 1993, 9, 61–63. [Google Scholar]
- Ferreira, M.S.; Borges, A.S. Some aspects of protozoan infections in immunocompromised patients-a review. Mem. Inst. Oswaldo. Cruz. 2002, 97, 443–457. [Google Scholar]
- Bosch-Driessen, L.E.; Berendschot, T.T.; Ongkosuwito, J.V.; Rothova, A. Ocular toxoplasmosis: clinical features and prognosis of 154 patients. Ophthalmology 2002, 109, 869–878. [Google Scholar]
- Paquet, C.; Yudin, M.H. Toxoplasmosis in pregnancy: Prevention, screening, and treatment. J. Obstet. Gynaecol. Can. 2013, 35, 78–79. [Google Scholar]
- Wallon, M.; Kodjikian, L.; Binquet, C.; Garweg, J.; Fleury, J.; Quantin, C.; Peyron, F. Long-term ocular prognosis in 327 children with congenital toxoplasmosis. Pediatrics 2004, 113, 1567–1572. [Google Scholar]
- Silveira, C.; Belfort, R.; Muccioli, C.; Holland, G.N.; Victora, C.G.; Horta, B.L.; Fei, Y.; Nussenblatt, R.B. The effect of long-term intermittent trimethoprim/sulfamethoxazole treatment on recurrences of toxoplasmic retinochoroiditis. Am. J. Ophthalmol. 2002, 134, 41–46. [Google Scholar]
- Yazici, A.; Ozdal, P.C.; Taskintuna, I.; Kavuncu, S.; Koklu, G. Trimethoprim/Sulfamethoxazole and azithromycin combination therapy for ocular toxoplasmosis. Ocul. Immunol. Inflamm. 2009, 17, 289–291. [Google Scholar]
- Dibbern, D.; Montanaro, A. Allergies to sulfonamide antibiotics and sulfur-containing drugs. Ann. Allerg. Asthma Immunol. 2008, 100, 91–101. [Google Scholar]
- Kongsaengdao, S.; Samintarapanya, K.; Oranratnachai, K.; Prapakarn, W.; Apichartpiyakul, C. Randomized controlled trial of pyrimethamine plus sulfadiazine versus trimethoprim plus sulfamethoxazole for treatment of toxoplasmic encephalitis in AIDS patients. J. Int. Assoc. Physicians AIDS Care 2008, 7, 11–16. [Google Scholar]
- McLeod, R.; Khan, A.R.; Noble, G.A.; Latkany, P.; Jalbrzikowski, J.; Boyer, K. Severe sulfadiazine hypersensitivity in a child with reactivated congenital toxoplasmic chorioretinitis. Pediatr. Infect. Dis. J. 2006, 25, 270–272. [Google Scholar]
- Aspinall, T.V.; Joynson, D.H.; Guy, E.; Hyde, J.E.; Sims, P.F. The molecular basis of sulfonamide resistance in Toxoplasma gondii and implications for the clinical management of toxoplasmosis. J. Infect. Dis. 2002, 185, 1637–1643. [Google Scholar]
- Remington, J.S.; McLeod, R.; Thulliez, P.; Desmonts, G. Toxoplasmosis. In Infectious Diseases of the Fetus and Newborn Infant, 7th ed.; Remington, J., Klein, J., Eds.; Saunders: Philadelphia, PA, USA, 2011. [Google Scholar]
- Degerli, K.; Kilimcioglu, A.A.; Kurt, Ö.; Tamay, T.; Ozbilgin, A. Efficacy of azithromycin in a murine toxoplasmosis model, employing a Toxoplasma gondii strain from Turkey. Acta Trop. 2003, 88, 45–50. [Google Scholar]
- Kijlstra, A.; Jongert, E. Toxoplasma-safe meat: Close to reality? Trends Parasitol. 2009, 25, 18–22. [Google Scholar]
- Dial, S.; Kezouh, A.; Dascal, A.; Barkun, A.; Suissa, S. Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile infection. CMAJ 2008, 179, 767–772. [Google Scholar]
- Megged, O.; Shalit, I.; Yaniv, I.; Stein, J.; Fisher, S.; Levy, I. Breakthrough cerebral toxoplasmosis in a patient receiving atovaquone prophylaxis after a hematopoietic stem cell transplantation. Pediatr. Transplant. 2008, 12, 902–905. [Google Scholar]
- Montoya, J.; Remington, J. Management of Toxoplasma gondii infection during pregnancy. Clin. Infect. Dis. 2008, 47, 554–566. [Google Scholar]
- Liesen, A.P.; de Aquino, T.M.; Carvalho, C.S.; Lima, V.T.; de Araújo, J.M.; de Lima, J.G.; de Faria, A.R.; de Melo, E.J.T.; Alves, A.J.; Alves, E.W.; et al. Synthesis and evaluation of anti-Toxoplasma gondii and antimicrobial activities of thiosemicarbazides, 4-thiazolidinones and 1,3,4-thiadiazoles. Eur. J. Med. Chem. 2010, 45, 3685–3691. [Google Scholar]
- Siwek, A.; Świderek, K.; Jankowski, S. Problems with molecular mechanics implementations on the example of 4-benzoyl-1-(4-methyl-imidazol-5-yl)-carbonyl-thiosemicarbazide. J. Mol. Model. 2012, 18, 843–849. [Google Scholar]
- Plech, T.; Wujec, M.; Siwek, A.; Kosikowska, U.; Malm, A. Synthesis and antimicrobial activity of thiosemicarbazides, s-triazoles and their Mannich bases bearing 3-chlorophenyl moiety. Eur. J. Med. Chem. 2011, 46, 241–248. [Google Scholar]
- Derouin, F.; Chastang, C. In vitro effects of folate inhibitors on Toxoplasma gondii. Antimicrob. Agents Chemother. 1989, 33, 1753–1759. [Google Scholar]
- Meneceur, P.; Bouldouyre, M.A.; Aubert, D.; Villena, I.; Menotti, J.; Sauvage, V.; Garin, J.F.; Derouin, F. In vitro susceptibility of various genotypicstrains of Toxoplasma gondii to pyrimethamine, sulfadiazine, and atovaquone. Antimicrob. Agents Chemother. 2008, 52, 1269–1277. [Google Scholar]
- Doliwa, C.; Escotte-Binet, S.; Aubert, D.; Velard, F.; Schmid, A.; Geers, R.; Villena, I. Induction of sulfadiazine resistance in vitro in Toxoplasma gondii. Exp. Parasitol. 2013, 133, 131–136. [Google Scholar]
- De Oliveira, T.C.; Silva, D.A.; Rostkowska, C.; Béla, S.R.; Ferro, E.A.; Magalhães, P.M.; Mineo, J.R. Toxoplasma gondii: Effects of Artemisia annua L. on susceptibility to infection in experimental models in vitro and in vivo. Exp. Parasitol. 2009, 122, 233–241. [Google Scholar]
- Van der Ven, A.J.; Schoondermark-van de Ven, E.M.; Camps, W.; Melchers, W.J.; Koopmans, P.P.; van der Meer, J.W.; Galama, J.M. Anti-toxoplasma effect of pyrimethamine, trimethoprim and sulphonamides alone and in combination: Implications for therapy. J. Antimicrob. Chemother. 1996, 38, 75–80. [Google Scholar]
- Jin, C.; Jung, S.Y.; Kim, S.Y.; Song, H.O.; Park, H. Simple and efficient model systems of screening anti-Toxoplasma drugs in vitro. Expert. Opin. Drug Discov. 2012, 7, 195–205. [Google Scholar]
- Alvarez, F.; Ghérardi, A.; Nebois, P.; Sarciron, M.E.; Pétavy, A.F.; Walchshofer, N. Benzimidazole-4,7-diones as inhibitors of protozoal (Toxoplasma gondii) purine nucleoside phosphorylase. Bioorg. Med. Chem. Lett. 2002, 12, 977–979. [Google Scholar]
- Kim, Y.A.; Rawal, R.K.; Yoo, J.; Sharon, A.; Jha, A.K.; Chu, C.K.; Rais, R.H.; Al Safarjalani, O.N.; Naguib, F.N.M.; Kouni, M.H. Structure-activity relationships of carbocyclic 6-benzylthioinosine analogues as subversive substrates of Toxoplasma gondii adenosine kinase. Bioorg. Med. Chem. 2010, 18, 3403–3412. [Google Scholar]
- Rosowsky, A.; Chen, H.; Fua, H.; Queener, S.F. Synthesis of new 2,4-diaminopyrido[2,3-d]pyrimidine and 2,4-diaminopyrrolo[2,3-d]pyrimidine inhibitors of pneumocystis carinii, Toxoplasma gondii, and Mycobacterium avium dihydrofolate reductase. Bioorg. Med. Chem. 2003, 11, 59–67. [Google Scholar]
- Rosowsky, A.; Forsch, R.A; Queener, S.F. 2,4-Diaminopyrido[3,2-d]pyrimidine inhibitors of dihydrofolate reductase from Pneumocystis carinii and Toxoplasma gondii. J. Med. Chem. 1995, 38, 2615–2620. [Google Scholar]
- Ojo, K.K.; Larson, E.T.; Keyloun, K.R.; Castaneda, L.J.; de Rocher, A.E.; Inampudi, K.K.; Kim, J.E.; Arakaki, T.L.; Murphy, R.C.; Zhang, L.; et al. Toxoplasma gondii calcium-dependent protein kinase 1 is a target for selective kinase inhibitors. Nat. Struct. Mol. Biol. 2010, 17, 602–607. [Google Scholar]
- Murphy, R.C.; Ojo, K.K.; Larson, E.T.; Castellanos-Gonzalez, A.; Perera, B.G.K.; Keyloun, K.R.; Kim, J.E.; Bhandari, J.G.; Muller, N.R.; Verlinde, C.L.M.J.; et al. Discovery of potent and selective inhibitors of calcium-dependent protein kinase 1 (CDPK1) from C. parvum and T. gondii. ACS Med. Chem. Lett. 2010, 1, 331–335. [Google Scholar]
- Sugi, T.; Kato, K.; Kobayashi, K.; Watanabe, S.; Kurokawa, H.; Gong, H.; Pandey, K.; Takemae, H.; Akashi, H. Use of the kinase inhibitor analog 1NM-PP1 reveals a role for Toxoplasma gondii CDPK1 in the invasion step. Eukaryot. Cell 2010, 9, 667–670. [Google Scholar]
- Zhang, Z.; Ojo, K.K.; Johnson, S.M.; Larson, E.T.; He, P.; Geiger, J.A.; Castellanos-Gonzalez, A.; White, A.C., Jr.; Parsons, M.; Merritt, E.A.; et al. Benzoylbenzimidazole-based selective inhibitors targeting Cryptosporidium parvum and Toxoplasma gondii calcium-dependent protein kinase-1. Bioorg. Med. Chem. Lett. 2012, 22, 5264–5267. [Google Scholar]
- Cai, G.; Deng, L.; Xue, J.; Moreno, S.N.J.; Striepen, B.; Song, Y. Expression, characterization and inhibition of Toxoplasma gondii 1-deoxy-D-xylulose-5-phosphate reductoisomerase. Bioorg. Med. Chem. Lett. 2013, 23, 2158–2161. [Google Scholar]
- Hunter, W.N. The non-mevalonate pathway of isoprenoid precursor biosynthesis. J. Biol. Chem. 2007, 282, 21573–21577. [Google Scholar]
- Singh, N.; Cheve, G.; Avery, M.A.; McCurdy, C.R. Targeting the methyl erythritol phosphate (MEP) pathway for novel antimalarial, antibacterial and herbicidal drug discovery: Inhibition of 1-deoxy-d-xylulose-5-phosphate reductoisomerase (DXR) enzyme. Curr. Pharm. Des. 2007, 13, 1161–1177. [Google Scholar]
- Cheng, G.; Muench, S.P.; Zhou, Y.; Afanador, G.A.; Mui, E.J.; Fomovska, A.; Lai, B.S.; Prigge, S.T.; Woods, S.; Roberts, C.W.; et al. Design, synthesis, and biological activity of diaryl ether inhibitors of Toxoplasma gondii enoyl reductase. Bioorg. Med. Chem. Lett. 2013, 23, 2035–2043. [Google Scholar]
- Kramer, B.; Rarey, M.; Lengauer, T. Evaluation of the FLEXX incremental construction algorithm for protein-ligand docking. Proteins 1999, 37, 228–241. [Google Scholar]
- LeadIT, version 2.1.0; BioSolveIT GmbH: St. Augustin, Germany, 2012.
- Siwek, A.; Stączek, P.; Stefańska, J. Synthesis and structure-activity relationship studies of 4-arylthiosemicarbazides as topoisomerase IV inhibitors with Gram-positive antibacterial activity. Search for molecular basis of antibacterial activity of thiosemicarbazides. Eur. J. Med. Chem. 2011, 46, 5717–5726. [Google Scholar]
- Siwek, A.; Stączek, P.; Wujec, M.; Stefańska, J.; Kosikowska, U.; Malm, A.; Jankowski, S.; Paneth, P. Biological and docking studies of topoisomerase IV inhibition by thiosemicarbazides. J. Mol. Model. 2011, 17, 2297–2303. [Google Scholar]
- Andersonm, V.E.; Gootz, T.D.; Osheroff, N. Topoisomerase IV Catalysis and the Mechanism of Quinolone Action. J. Biol. Chem. 1998, 273, 17879–17885. [Google Scholar]
- Nath, T.G.S.; Husain, S.; Srinivasan, V.R. Synthesis and reactivity of 4,5-disubstituted 3-chloro-1,2,4-triazoles and their methylsulfonyl analogs. Indian J. Chem. Sec. B 1977, 15B, 341–346. [Google Scholar]
- Siwek, A.; Stefańska, J.; Dzitko, K.; Ruszczak, A. Antifungal effect of 4-arylthiosemicarbazides against Candida species. Search for molecular basis of antifungal activity of thiosemicarbazide derivatives. J. Mol. Model. 2012, 18, 4159–4170. [Google Scholar]
- Chen, H.-L.; Guo, Z.-F.; Lu, Z.-L. Controlling ion-sensing specificity of N-amidothioureas: From anion-selective sensors to highly Zn2+-selective sensors by tuning electronic effects. Org. Lett. 2012, 14, 5070–5073. [Google Scholar]
- Goldfarb, D.S. Method Using Lifespan-Altering Compounds For Altering The Lifespan Of Eukaryotic Organisms, and Screening for Such Compounds. U.S. Patent US20090163545 A1, 25 June 2009. [Google Scholar]
- Siwek, A.; Wujec, M.; Dobosz, M.; Jagiello-Wojtowicz, E.; Kleinrok, A.; Chodkowska, A.; Paneth, P. Chemical and pharmacological properties of 3-(thiophen-2-yl)-4-substituted-Δ2-1,2,4-triazoline-5-thiones. Phosphorus Sulfur 2008, 183, 2669–2677. [Google Scholar]
- Siwek, A.; Wujec, M.; Stefanska, J.; Paneth, P. Antimicrobial properties of 4-aryl-3-(2-methyl-furan-3-yl)-Δ2-1,2,4-triazoline-5-thiones. Phosphorus Sulfur 2009, 184, 3149–3159. [Google Scholar]
- Burg, J.L.; Grover, C.M.; Pouletty, P.; Boothroyd, J.C. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J. Clin. Microbiol. 1989, 27, 1787–1792. [Google Scholar]
- Wahab, T.; Edvinsson, B.; Palm, D.; Lindh, J. Comparison of the AF146527 and B1 repeated elements, two real-time PCR targets used for detection of Toxoplasma gondii. J. Clin. Microbiol. 2010, 48, 591–592. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial disc Susceptibility Tests, Approved Standard M2-A9. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2006. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard M7-A7; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2006. [Google Scholar]
- Hyperchem, version 8.0.3; HyperCube Inc.: Gainsville, FL, USA, 2007.
- Singh, U.C.; Kollman, P.A. An approach to computing electrostatic charges for molecules. J. Comp. Chem. 1984, 5, 129–145. [Google Scholar]
- Frisch, M.J. Gaussian 09; revision A.02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Sample Availability: Samples of the tested compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzitko, K.; Paneth, A.; Plech, T.; Pawełczyk, J.; Stączek, P.; Stefańska, J.; Paneth, P. 1,4-Disubstituted Thiosemicarbazide Derivatives are Potent Inhibitors of Toxoplasma gondii Proliferation. Molecules 2014, 19, 9926-9943. https://doi.org/10.3390/molecules19079926
Dzitko K, Paneth A, Plech T, Pawełczyk J, Stączek P, Stefańska J, Paneth P. 1,4-Disubstituted Thiosemicarbazide Derivatives are Potent Inhibitors of Toxoplasma gondii Proliferation. Molecules. 2014; 19(7):9926-9943. https://doi.org/10.3390/molecules19079926
Chicago/Turabian StyleDzitko, Katarzyna, Agata Paneth, Tomasz Plech, Jakub Pawełczyk, Paweł Stączek, Joanna Stefańska, and Piotr Paneth. 2014. "1,4-Disubstituted Thiosemicarbazide Derivatives are Potent Inhibitors of Toxoplasma gondii Proliferation" Molecules 19, no. 7: 9926-9943. https://doi.org/10.3390/molecules19079926